|
Diimide
Diimide, also called diazene or diimine, is a compound having the formula (NH)2. It exists as two geometric isomers, ''E'' (''trans'') and ''Z'' (''cis''). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is an example of an organic diazene. Synthesis A traditional route to diimide involves oxidation of hydrazine with hydrogen peroxide or air. Alternatively the hydrolysis of diethyl azodicarboxylate or azodicarbonamide affords diimide: :(NCOOH)2 → (NH)2 + 2 CO2 Nowadays, diimide is generated by thermal decomposition of 2,4,6‐triisopropylbenzenesulfonylhydrazide. Because of its instability, diimide is generated and used ''in-situ''. A mixture of both the ''cis'' (''Z-'') and ''trans'' (''E-'') isomers is produced. Both isomers are unstable, and they undergo a slow interconversion. The ''trans'' isomer is more stable, but the ''cis'' isomer is the one that reacts with unsaturated substrates, therefore the equilibrium between them shi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing polymer foams, but applications also include its uses as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for in- space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. the world hydrazine hydrate market amounted to $350 million. About two million tons of hydrazine hydrate were used in foam blowing agents in 2015. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons. Process Hydrogenation has three components, the unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same catalysts and conditions that are used for hydrogenation reactions can also lead to isomerization of the alkenes from cis t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azobenzene
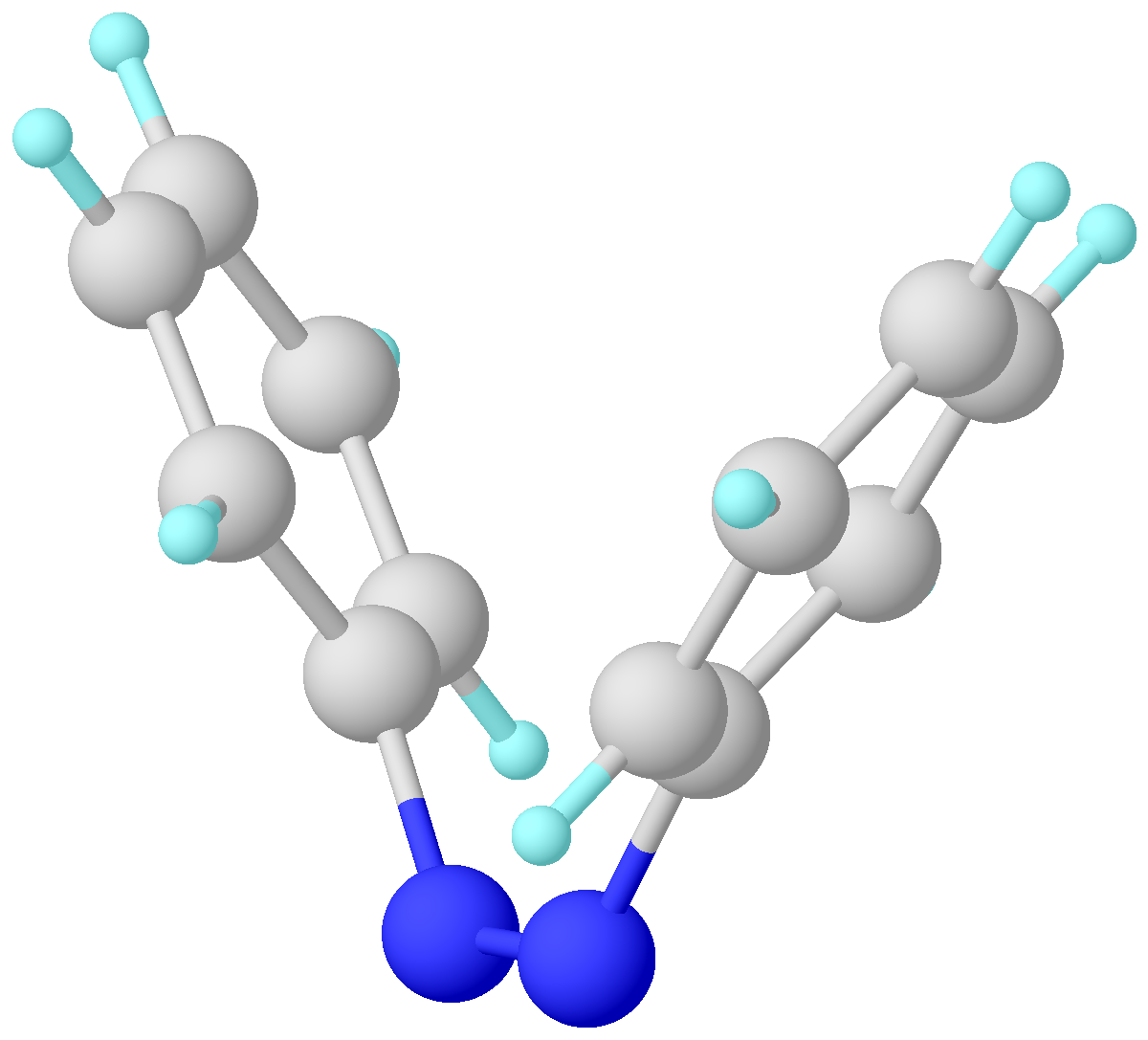
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Structure and synthesis ''trans''-Azobenzene is planar. The N-N distance is 1.189 Å. ''cis''-Azobenzene is nonplanar with a C-N=N-C dihedral angle of 173.5°. The N-N distance is 1.251 Å. Azobenzene was first described by Eilhard Mitscherlich in 1834. Yellowish-red crystalline flakes of azobenzene were obtained in 1856. Its original preparation is similar to the modern one. According to the 1856 method, nitrobenzene is reduced by iron filings in the presence of acetic acid. In the modern synthesis, zinc is the reductant in the presence of a base. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isodiazene
In organic chemistry, an isodiazene, also known by the incorrectly constructed (but commonly used) name 1,1-diazene or systematic name diazanylidene, is an organic derivative of the parent isodiazene (H2N+=N–, also called 1,1-diimide) with general formula R1R2N+=N–. The functional group has two major resonance forms, a diazen-2-ium-1-ide form, and an aminonitrene form: Although isodiazenes are formally isoelectronic with ketones and aldehydes, the reactivity of this exotic functional group is very different. They are generally prepared by oxidation of the hydrazine (R2N–NH2), reduction of the 1,1-diazene oxide (R2N–N=O), 1,1-elimination of MX from R2N–NMX (M = Na, K; X = SO2Ar), or treatment of secondary amines with Angeli's salt, Na2N2O3, in the presence of acid. Isodiazenes participate in cycloaddition reactions with alkenes to generate ''N''-aminoaziridines. In the absence of other reactants, they undergo reactions in which N2 is eliminated to give an organic res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triazene
Triazene is an unsaturated inorganic compound having the chemical formula N3 H3. It has one double bond and is the second-simplest member of the azene class of hydronitrogen compounds, after diimide. Triazenes are a class of organic compounds containing the functional group -N(H)-N=N-. Triazene, possibly along with its isomer triimide (HNNHNH), has been synthesized in electron-irradiated ices of ammonia and ammonia/ dinitrogen and detected in the gas phase after sublimation.Forstel, Tsegaw, Maksyutenko, Mebel, Sander, & Kaiser. "On the formation of N3H3 isomers in irradiated ammonia bearing ices: Triazene (H2NNNH) or Triimide (HNHNNH)", ''ChemPhysChem'', 2016, 17, 2726. References External links *IUPAC The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ... Gold Bookbr>definiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Compound
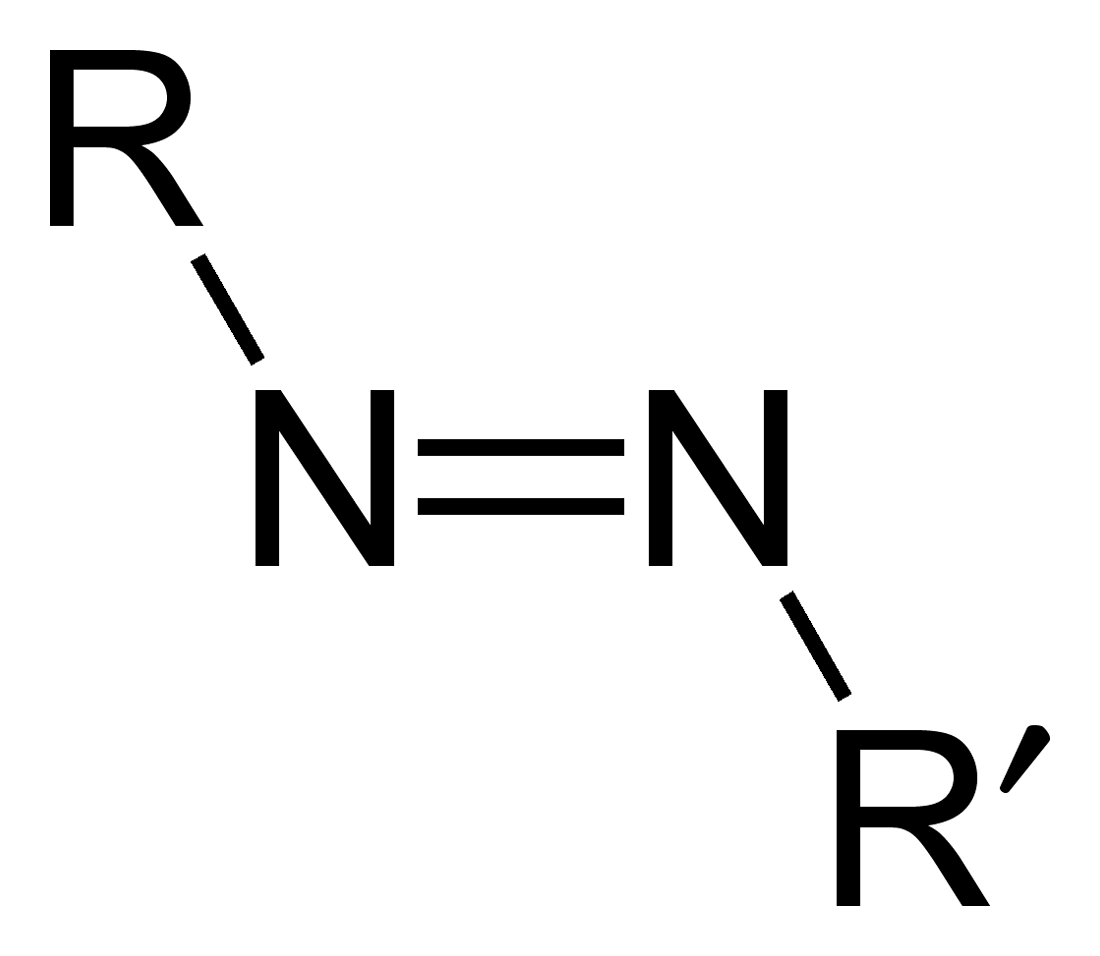
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diaz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Compounds
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diazon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FNNF
Dinitrogen difluoride is a chemical compound with the formula N2F2. It is a gas at room temperature, and was first identified in 1952 as the thermal decomposition product of the azide N3F. It has the structure F−N=N−F and exists in both a ''cis''- and ''trans''-form. Isomers The ''cis'' configuration lies in a C2v symmetry and the ''trans''-form has a symmetry of C2h. These isomers are thermally interconvertible but can be separated by low temperature fractionation. The ''trans''-form is less thermodynamically stable but can be stored in glass vessels. The ''cis''-form attacks glass over a time scale of about 2 weeks to form silicon tetrafluoride and nitrous oxide: :2 N2F2 + SiO2 → SiF4 + 2 N2O Preparation Most preparations of dinitrogen difluoride give mixtures of the two isomers, but they can be prepared independently. An aqueous method involves ''N'',''N''-difluorourea with concentrated potassium hydroxide. This gives a 40% yield with three times more o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Hydrides
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many industrially important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Relative Bond Strength Order
Relative may refer to: General use *Kinship and family, the principle binding the most basic social units society. If two people are connected by circumstances of birth, they are said to be ''relatives'' Philosophy *Relativism, the concept that points of view have no absolute truth or validity, having only relative, subjective value according to differences in perception and consideration, or relatively, as in the relative value of an object to a person *Relative value (philosophy) Economics *Relative value (economics) Popular culture Film and television * ''Relatively Speaking'' (1965 play), 1965 British play * ''Relatively Speaking'' (game show), late 1980s television game show * ''Everything's Relative'' (episode)#Yu-Gi-Oh! (Yu-Gi-Oh! Duel Monsters), 2000 Japanese anime ''Yu-Gi-Oh! Duel Monsters'' episode *'' Relative Values'', 2000 film based on the play of the same name. *''It's All Relative'', 2003-4 comedy television series *''Intelligence is Relative'', tag line for t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |