|
Dicobalt Hexacarbonyl Acetylene Complex
Dicobalt hexacarbonyl acetylene complexes are a family of In organocobalt compounds with the formula . A large variety of R groups are tolerated. They are red compounds that are soluble in organic solvents. They arise from the reaction of alkynes and dicobalt octacarbonyl: : According to X-ray crystallography, the two Co atoms and two alkyne carbons form the vertices of a distorted tetrahedron. The C-C distance for the bridging alkyne ligand is 1.33 Å, and the Co-Co distance is 2.47 Å. The core has C2v symmetry.{{cite journal , doi=10.1021/om00161a029, title=Diastereoselective Ligand and Vertex Substitutions in Bimetallic Bridged Alkyne Clusters: X-Ray Crystal Structure of .mu.2-(endo-2-Propynylborneol)hexacarbonyldicobalt , year=1990 , last1=d'Agostino , first1=Michael F. , last2=Frampton , first2=Christopher S. , last3=McGlinchey , first3=Michael J. , journal=Organometallics , volume=9 , issue=11 , pages=2972–2984 The structure is related to that of methylidynetricoba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Co2(C2Me2)(CO)6
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organocobalt Compound
Organocobalt chemistry is the chemistry of organometallic compounds containing a carbon to cobalt chemical bond. Organocobalt compounds are involved in several organic reactions and the important biomolecule vitamin B12, vitamin B12 has a cobalt-carbon bond. Many organocobalt compounds exhibit useful catalytic properties, the preeminent example being dicobalt octacarbonyl. Alkyl complexes Most fundamental are the cobalt complexes with only alkyl ligands. Examples include Co(4-norbornyl)4 and its cation. Alkylcobalt is represented by vitamin B12, vitamin B12 and related enzymes. In methylcobalamin the ligand is a methyl group, which is electrophilic. in vitamin B12, the alkyl ligand is an adenosyl group. Related to vitamin B12 are cobalt porphyrins, dimethylglyoxime, dimethylglyoximates, and related complexes of Schiff base ligands. These synthetic compounds also form alkyl derivatives that undergo diverse reactions reminiscent of the biological processes. The weak cobalt(III) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicobalt Octacarbonyl
Dicobalt octacarbonyl is an organocobalt compound with composition . This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the parent member of a family of hydroformylation catalysts. Each molecule consists of two cobalt atoms bound to eight carbon monoxide ligands, although multiple structural isomers are known. Some of the carbonyl ligands are labile. Synthesis, structure, properties Dicobalt octacarbonyl an orange-colored, pyrophoric solid. It is synthesised by the high pressure carbonylation of cobalt(II) salts: : The preparation is often carried out in the presence of cyanide, converting the cobalt(II) salt into a pentacyanocobaltate(II) complex that reacts with carbon monoxide to yield . Acidification produces cobalt tetracarbonyl hydride, , which degrades near room temperature to dicobalt octacarbonyl and hydrogen. It can also be prepared by heating cobalt me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring the angles and intensities of the X-ray diffraction, a crystallography, crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and the positions of the atoms, as well as their chemical bonds, crystallographic disorder, and other information. X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences between various materials, especially minerals and alloys. The method has also revealed the structure and function of many biological molecules, including vitamins, drugs, proteins and nucleic acids such as DNA. X-ray crystall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne Complex
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 118 picometers (for C2H2) is much shorter than the C=C distance in alkenes (132 pm, for C2H4) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symmetry Group
In group theory, the symmetry group of a geometric object is the group of all transformations under which the object is invariant, endowed with the group operation of composition. Such a transformation is an invertible mapping of the ambient space which takes the object to itself, and which preserves all the relevant structure of the object. A frequent notation for the symmetry group of an object ''X'' is ''G'' = Sym(''X''). For an object in a metric space, its symmetries form a subgroup of the isometry group of the ambient space. This article mainly considers symmetry groups in Euclidean geometry, but the concept may also be studied for more general types of geometric structure. Introduction We consider the "objects" possessing symmetry to be geometric figures, images, and patterns, such as a wallpaper pattern. For symmetry of physical objects, one may also take their physical composition as part of the pattern. (A pattern may be specified formally as a scalar field, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylidynetricobaltnonacarbonyl
Methylidynetricobaltnonacarbonyl is an Organometallic chemistry, organometallic cobalt cluster with the chemical formula Co3(CO)9CH that contains a metal carbonyl core with the Methylidyne group, methylidyne ligand, first discovered in the late 1950s. A variety of substituents can be added to the methylidyne group to form derivatives of the parent compound that have unique spectroscopic properties and reactivity. This page will explore the discovery and synthesis of methylidynetricobaltnonacarbonyl, the structure and bonding of the parent compound, as well as some examples reactivity and catalysis with the cluster. Synthesis Methylidynetricobaltnonacarbonyl and derivatives were discovered in the late 1950s by Markby and Wender by reactions of the alkyne complexes with acids. The structure of the cluster was however misformulated. In 1962, the class of compounds were properly formulated methylidynetricobaltnonacarbonyl as well as several derivatives. The synthetic procedure deve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetracobalt Dodecacarbonyl
Tetracobalt dodecacarbonyl is the chemical compound with the formula Co4(CO)12. It is a black crystalline compound that is insoluble in water and easily oxidized by air. It is an example of a metal carbonyl cluster. Synthesis and structure This compound is synthesized by decarbonylation of Co2(CO)8. :2 Co2(CO)8 → Co4(CO)12 + 4 CO The molecule consists of a tetrahedral Co4 core, but the molecular symmetry is C3v. Three carbonyl ligands are bridging ligand In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually r ...s and nine are terminal. The average Co-Co distance is 2.499 Å, the average C-O bond length is 1.133 Å, and the average Co-C-O angle is 177.5°. Rh4(CO)12 adopts the same C3v structure but Ir4(CO)12 has perfect Td symmetry with no bridging CO ligands groups. The Rh4 and Ir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
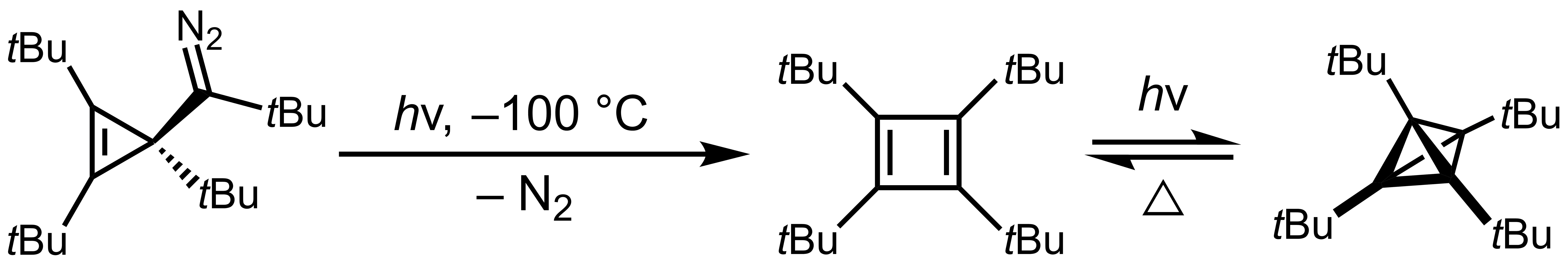
Tetrahedrane
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized . However, a number of derivatives have been prepared. In a more general sense, the term ''tetrahedranes'' is used to describe a class of molecules and ions with related structure, e.g. white phosphorus. C4 tetrahedranes Tetrahedrane () is one of the possible platonic hydrocarbons and has the IUPAC name tricyclo .1.0.02,4utane. Unsubstituted tetrahedrane remains elusive, although predicted kinetically stable. One strategy that has been explored (but thus far failed) is reaction of propene with atomic carbon. Contrariwise, several organic compounds with the tetrahedrane core are known. All have multiply bulky substituents, ''tert''-butyl (''t''-Bu) or larger. Locking a tetrahedrane molecule inside a fullerene has only been attempted ''in silico''. All known syntheses have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |