|
Diacid
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic.Boy Cornils, Peter Lappe "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry 2014, Wiley-VCH, Weinheim. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids. Dicarboxylic acids are usually colorless solids. A wide variety of dicarboxylic acids are used in industry. Adipic acid, for example, is a precursor to certain kinds of nylon. A wide variety of dicarboxylic acids are found in nature. Aspartic acid and glutamic acid are two amino acids found in all life. Succinic and fumaric acids are essential for metabolism. A large inventory of derivatives are known including many mono- and diesters, amides, etc. Partial list of saturated dicarboxylic acids Some common or illustrative examples : Unsa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
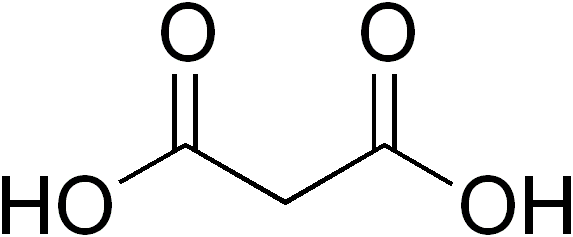
Succinic Acid
Succinic acid () is a dicarboxylic acid with the chemical formula (CH2)2(CO2H)2. In living organisms, succinic acid takes the form of an anion, succinate, which has multiple biological roles as a metabolic intermediate being converted into fumarate by the enzyme succinate dehydrogenase in complex 2 of the electron transport chain which is involved in making ATP, and as a signaling molecule reflecting the cellular metabolic state. Succinate is generated in mitochondria via the tricarboxylic acid (TCA) cycle. Succinate can exit the mitochondrial matrix and function in the cytoplasm as well as the extracellular space, changing gene expression patterns, modulating epigenetic landscape or demonstrating hormone-like signaling. As such, succinate links cellular metabolism, especially ATP formation, to the regulation of cellular function. Dysregulation of succinate synthesis, and therefore ATP synthesis, happens in some genetic mitochondrial diseases, such as Leigh syndrome, and Me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thapsic Acid
Thapsic acid (hexadecanedioic acid) is a naturally occurring dicarboxylic acid with the formula C16H30O4. The name is derived from ''Thapsia'', the Latin name for a Mediterranean perennial whose roots contain thapsic acid. It has a role as a human metabolite.Pettersen JE, Aas M. Subcellular localization of hexadecanedioic acid activation in human liver. J Lipid Res. 1974 Nov;15(6):551–556. It is the conjugate acid A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the rever ... of hexadecanedioate. References Dicarboxylic acids {{OrganicAcid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brassylic Acid
Brassylic acid is an organic compound with chemical formula . A white solid, it is the C13-dicarboxylic acid. It is prepared by oxidation of erucic acid, which is abundant in some seed oils. Pelargonic acid is the coproduct. In the industrial setting, brassylic acid is used to produce specialty nylon Nylon is a family of synthetic polymers characterised by amide linkages, typically connecting aliphatic or Polyamide#Classification, semi-aromatic groups. Nylons are generally brownish in color and can possess a soft texture, with some varieti ...s, e.g. nylon 1313. References {{Navbox linear saturated dicarboxylic acids Dicarboxylic acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dodecanedioic Acid
Dodecanedioic acid (DDDA) is a dicarboxylic acid with the formula . A white solid, the compound finds a variety of applications ranging from polymers to materials. The unbranched compound is the most commonly encountered C12 dicarboxylic acid. Production DDDA has traditionally been produced from butadiene using a multi-step chemical process. Butadiene is first converted to cyclododecatriene through cyclotrimerization. The triene is then hydrogenated to cyclododecane. Autoxidation by air in the presence of boric acid gives a mixture of cyclodecanol and the cyclododecanone. In the final step, this mixture oxidized to the diacid using nitric acid. An alternative route involves ozonolysis of cyclododecene. : Biological process Paraffin wax can be converted into DDDA on a laboratory scale with a special strain of ''Candida tropicalis'' yeast in a multi-step process. Renewable plant-oil feedstocks sourced from switchgrass could also be used to produce DDDA. Uses DDDA is used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sebacic Acid
Sebacic acid is a naturally occurring dicarboxylic acid with the chemical formula . It is a white flake or powdered solid. ''Sebaceus'' is Latin for tallow candle, ''sebum'' is Latin for tallow, and refers to its use in the manufacture of candles. Sebacic acid is a derivative of castor oil. In the industrial setting, sebacic acid and its homologues such as azelaic acid can be used as a monomer for nylon 610, plasticizers, lubricants, hydraulic fluids, cosmetics, candles, etc. It can be used as a surfactant in the lubricating oil industry to increase the antirust properties of lubricating oils on metals. Production and reactions Sebacic acid is produced from castor oil by cleavage of ricinoleic acid, which is obtained from castor oil. Octanol and glycerin are byproducts. It can also be obtained from decalin via the a hydroperoxide, which rearranges to give a hydroxycyclodecanone, which dehydrates to give cyclodecenone, a precursor to sebacic acid. Sebacic acid has also been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azelaic Acid
Azelaic acid (AzA), or nonanedioic acid, is an organic compound with the formula HOOC(CH2)7carboxylic acid, COOH. This saturated dicarboxylic acid exists as a white powder. It is found in wheat, rye, and barley. It is a precursor to diverse industrial products including polymers and plasticizers, as well as being a component of several hair and skin conditioners. AzA inhibits tyrosinase. Production Azelaic acid is industrially produced by the ozonolysis of oleic acid. The side product is nonanoic acid. It is produced naturally by ''Malassezia furfur'' (also known as ''Pityrosporum ovale''), a yeast that lives on normal skin. The bacterial degradation of Pelargonic acid, nonanoic acid gives azelaic acid. Biological function Plants biology In plants, azelaic acid serves as a "distress flare" involved in defense responses after infection. It serves as a signal that induces the accumulation of salicylic acid, an important component of a plant's defensive response. Human biology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,4-Cyclohexanedicarboxylic Acid
1,4-Cyclohexanedicarboxylic acid describes a pair of organic compounds with the formula . The CO2H groups are attached to the opposite carbon centers of the cyclohexane ring. These groups can be cis or trans. Other isomers of cyclohexanedicarboxylic acid are known, but the 1,4- isomers are of greatest interest, perhaps because they are obtainable from a commodity chemical. Specifically, hydrogenation of terephthalic acid affords the title compound: :. The trans isomer has been more heavily studied. It has been examined as a precursor to polycarbonate Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate ester, carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, toughness, tough materials, and some grades are optically transp ...s and as a building block for metal-organic frameworks. References {{DEFAULTSORT:Cyclohexanedicarboxylic acid, 1,4- Dicarboxylic acids Cyclohexanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Suberic Acid
Suberic acid, also octanedioic acid, is a dicarboxylic acid In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic.Boy Cornils, Peter Lappe "Dicarbox ..., with formula C8H14O4. It is a colorless crystalline solid used in drug syntheses and plastics manufacture. Its name is derived from the Latin word ''suber'' which means cork. References Dicarboxylic acids {{OrganicAcid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |