|
Cyclic Di-GMP
Cyclic di-GMP (also called cyclic diguanylate and c-di-GMP) is a second messenger used in signal transduction in a wide variety of bacteria. Cyclic di-GMP is not known to be used by archaea, and has only been observed in eukaryotes in ''Dictyostelium''. The biological role of cyclic di-GMP was first uncovered when it was identified as an allosteric activator of a cellulose synthase found in ''Gluconacetobacter xylinus'' in order to produce microbial cellulose. In structure, it is a cycle containing only two guanine bases linked by ribose and phosphate. Contact with surfaces increases c-di-GMP which increases transcription, translation, and post translation of exopolysaccharides (EPSs) and other extracellular polymeric substance matrix components (see the review by Jenal et al 2017). In bacteria, certain signals are communicated by synthesizing or degrading cyclic di-GMP. Cyclic di-GMP is synthesized by proteins with diguanylate cyclase activity. These proteins typically have a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
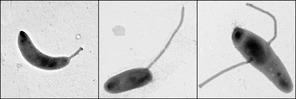
Caulobacter Crescentus
''Caulobacter crescentus'' is a Gram-negative, oligotrophic bacterium widely distributed in fresh water lakes and streams. The taxon is more properly known as ''Caulobacter vibrioides'' (Henrici and Johnson 1935). ''C. crescentus'' is an important model organism for studying the regulation of the cell cycle, asymmetric cell division, and cellular differentiation. ''Caulobacter'' daughter cells have two very different forms. One daughter is a mobile "swarmer" cell that has a single flagellum at one cell pole that provides swimming motility for chemotaxis. The other daughter, called the "stalked" cell, has a tubular stalk structure protruding from one pole that has an adhesive holdfast material on its end, with which the stalked cell can adhere to surfaces. Swarmer cells differentiate into stalked cells after a short period of motility. Chromosome replication and cell division only occurs in the stalked cell stage. ''C. crescentus'' derives its name from its crescent shape, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Di-GMP-II Riboswitch
Cyclic di-GMP-II riboswitches (also c-di-GMP-II riboswitches) form a class of riboswitches that specifically bind cyclic di-GMP, a second messenger used in multiple bacterial processes such as virulence, motility and biofilm formation. Cyclic di-GMP II riboswitches are structurally unrelated to cyclic di-GMP-I riboswitches, though they have the same function. Cyclic di-GMP-II riboswitches were discovered by bioinformatics, and are common in species within the class Clostridia and the genus ''Deinococcus''. They are also found in some other bacterial lineages. There is significant overlap between species that use cyclic di-GMP-I and cyclic di-GMP-II riboswitches, as both riboswitch classes are common in Clostridia. In '' Clostridium difficile'' strains, a cyclic di-GMP-II riboswitch is found adjacent to a group I catalytic intron. Group I introns are ribozymes that catalyze the splicing of the RNA molecule in which they are embedded. In the riboswitch-associated case, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Di-GMP-I Riboswitch
Cyclic di-GMP-I riboswitches are a class of riboswitch that specifically bind cyclic di-GMP, which is a second messenger that is used in a variety of microbial processes including virulence, motility and biofilm formation. Cyclic di-GMP-I riboswitches were originally identified by bioinformatics as a conserved RNA-like structure called the "GEMM motif". These riboswitches are present in a wide variety of bacteria, and are most common in Clostridia and certain varieties of Pseudomonadota. The riboswitches are present in pathogens such as '' Clostridium difficile'', ''Vibrio cholerae'' (which causes cholera) and ''Bacillus anthracis'' (which causes anthrax). '' Geobacter uraniumreducens'' is predicted to have 30 instances of this riboswitch in its genome. A bacteriophage that infects ''C. difficile'' is predicted to carry a cyclic di-GMP-I riboswitch, which it might use to detect and exploit the physiological state of bacteria that it infects. The discovery of this riboswitch cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Riboswitch
In molecular biology, a riboswitch is a regulatory segment of a messenger RNA molecule that binds a small molecule, resulting in a change in production of the proteins encoded by the mRNA. Thus, an mRNA that contains a riboswitch is directly involved in regulating its own activity, in response to the concentrations of its effector molecule. The discovery that modern organisms use RNA to bind small molecules, and discriminate against closely related analogs, expanded the known natural capabilities of RNA beyond its ability to code for proteins, catalyze reactions, or to bind other RNA or protein macromolecules. The original definition of the term "riboswitch" specified that they directly sense small-molecule metabolite concentrations. Although this definition remains in common use, some biologists have used a broader definition that includes other cis-regulatory RNAs. However, this article will discuss only metabolite-binding riboswitches. Most known riboswitches occur in bac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PAS Domain
A Per-Arnt-Sim (PAS) domain is a protein domain found in all kingdoms of life. Generally, the PAS domain acts as a molecular sensor, whereby small molecules and other proteins associate via binding of the PAS domain. Due to this sensing capability, the PAS domain has been shown as the key structural motif involved in protein-protein interactions of the circadian clock, and it is also a common motif found in signaling proteins, where it functions as a signaling sensor. Discovery PAS domains are found in a large number of organisms from bacteria to mammals. The PAS domain was named after the three proteins in which it was first discovered: * Per – period circadian protein * Arnt – aryl hydrocarbon receptor nuclear translocator protein * Sim – single-minded protein Since the initial discovery of the PAS domain, a large quantity of PAS domain binding sites have been discovered in bacteria and eukaryotes. A subset called PAS LOV proteins are responsive to oxygen, light a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EAL Domain
In molecular biology, the EAL domain is a conserved protein domain. It is found in diverse bacterial signalling proteins. It is named EAL after its conserved residues. The EAL domain may function as a diguanylate phosphodiesterase. The domain contains many conserved acidic residues that could participate in metal binding and might form the phosphodiesterase active site In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) a .... References {{InterPro content, IPR001633 Protein domains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Virulence
Virulence is a pathogen's or microorganism's ability to cause damage to a host. In most, especially in animal systems, virulence refers to the degree of damage caused by a microbe to its host. The pathogenicity of an organism—its ability to cause disease—is determined by its virulence factors. In the specific context of gene for gene systems, often in plants, virulence refers to a pathogen's ability to infect a resistant host. The noun ''virulence'' derives from the adjective ''virulent'', meaning disease severity. The word ''virulent'' derives from the Latin word ''virulentus'', meaning "a poisoned wound" or "full of poison." From an ecological standpoint, virulence is the loss of fitness induced by a parasite upon its host. Virulence can be understood in terms of proximate causes—those specific traits of the pathogen that help make the host ill—and ultimate causes—the evolutionary pressures that lead to virulent traits occurring in a pathogen strain. Virulent ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Motility
Motility is the ability of an organism to move independently, using metabolic energy. Definitions Motility, the ability of an organism to move independently, using metabolic energy, can be contrasted with sessility, the state of organisms that do not possess a means of self-locomotion and are normally immobile. Motility differs from mobility, the ability of an object to be moved. The term vagility encompasses both motility and mobility; sessile organisms including plants and fungi often have vagile parts such as fruits, seeds, or spores which may be dispersed by other agents such as wind, water, or other organisms. Motility is genetically determined, but may be affected by environmental factors such as toxins. The nervous system and musculoskeletal system provide the majority of mammalian motility. In addition to animal locomotion, most animals are motile, though some are vagile, described as having passive locomotion. Many bacteria and other microorganisms, and multicellu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
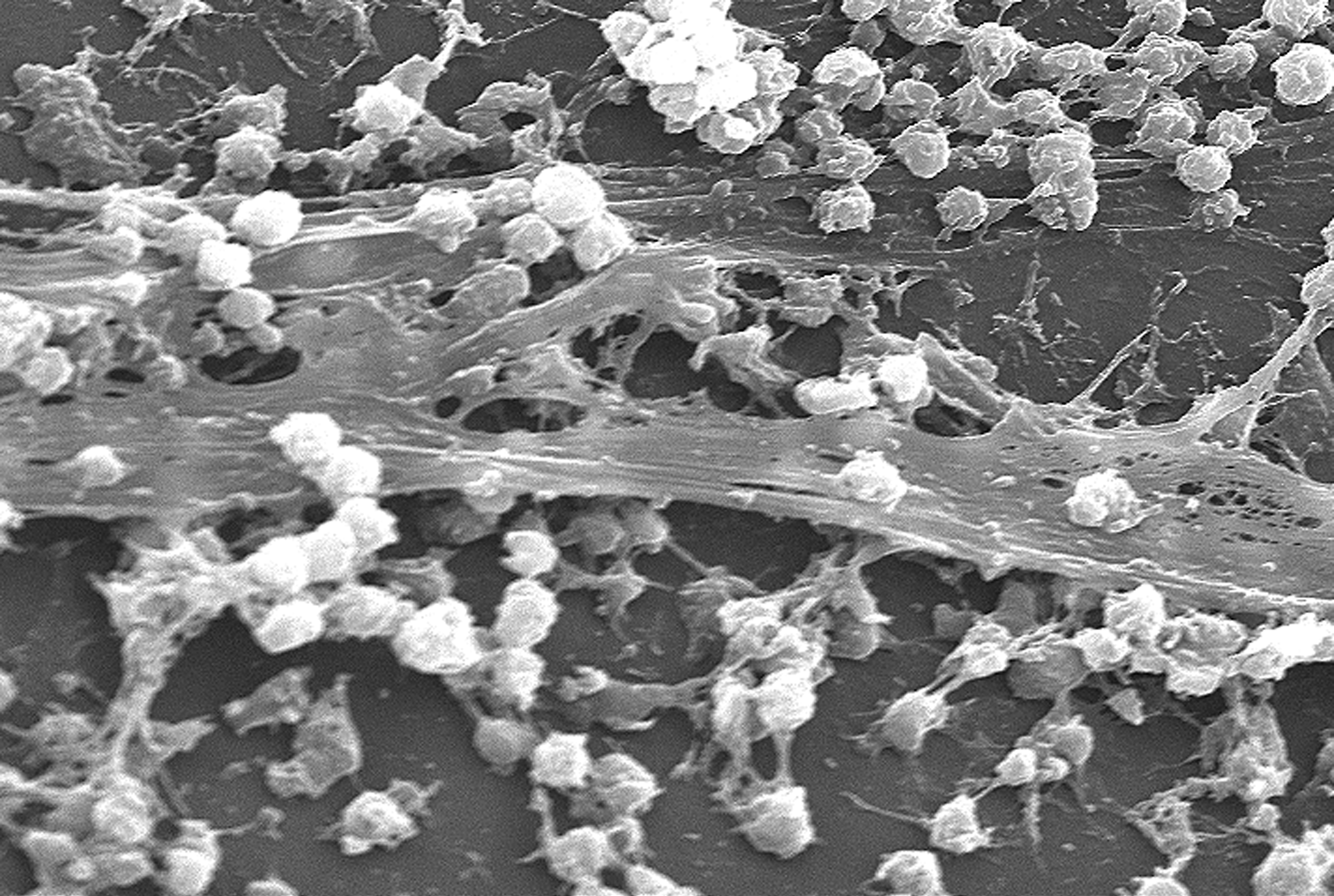
Biofilm
A biofilm comprises any syntrophic consortium of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular polymeric substances (EPSs). The cells within the biofilm produce the EPS components, which are typically a polymeric conglomeration of extracellular polysaccharides, proteins, lipids and DNA. Because they have three-dimensional structure and represent a community lifestyle for microorganisms, they have been metaphorically described as "cities for microbes". Biofilms may form on living or non-living surfaces and can be prevalent in natural, industrial, and hospital settings. They may constitute a microbiome or be a portion of it. The microbial cells growing in a biofilm are physiologically distinct from planktonic cells of the same organism, which, by contrast, are single cells that may float or swim in a liquid medium. Biofilms can form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphodiesterase
A phosphodiesterase (PDE) is an enzyme that breaks a phosphodiester bond. Usually, ''phosphodiesterase'' refers to cyclic nucleotide phosphodiesterases, which have great clinical significance and are described below. However, there are many other families of phosphodiesterases, including phospholipases C and D, autotaxin, sphingomyelin phosphodiesterase, DNases, RNases, and restriction endonucleases (which all break the phosphodiester backbone of DNA or RNA), as well as numerous less-well-characterized small-molecule phosphodiesterases. The cyclic nucleotide phosphodiesterases comprise a group of enzymes that degrade the phosphodiester bond in the second messenger molecules cAMP and cGMP. They regulate the localization, duration, and amplitude of cyclic nucleotide signaling within subcellular domains. PDEs are therefore important regulators of signal transduction mediated by these second messenger molecules. History These multiple forms (isoforms or subtypes) of phosphodies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |