|
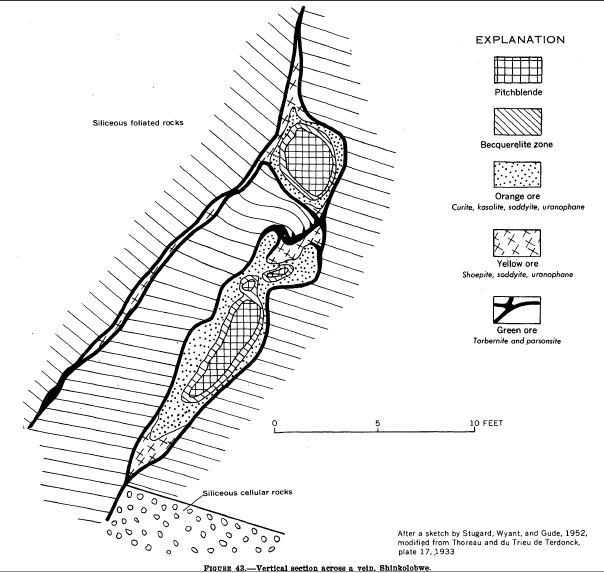
Curite
Curite is a lead uranium oxide mineral with formula: Pb3(UO2)8O8(OH)6·3(H2O). It is named after the physicists Marie and Pierre Curie Pierre Curie ( , ; 15 May 1859 – 19 April 1906) was a French physicist, a pioneer in crystallography, magnetism, piezoelectricity, and radioactivity. In 1903, he received the Nobel Prize in Physics with his wife, Marie Curie, and Henri Becq ..., who are both known for their work on radioactivity. The type locality is the Shinkolobwe Mine. References Lead minerals Oxide minerals Uranium(VI) minerals Orthorhombic minerals Minerals in space group 62 {{oxide-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shinkolobwe Mine
Shinkolobwe, or Kasolo, or Chinkolobew, or Shainkolobwe, was a radium and uranium mine in the Haut-Katanga Province of the Democratic Republic of the Congo (DRC), located 20 km west of Likasi (formerly Jadotville), 20 km south of Kambove, and about 145 km northwest of Lubumbashi. The mine produced the most economical uranium ore in the world and was used for the Manhattan Project and subsequent nuclear weapons produced by the United States in the 1940s and 50s. Before World War II, uranium extracted here was originally taken to Belgium to be processed; this supply was captured by the Wehrmacht in 1940 and subsequently used for the unsuccessful German nuclear program. The Shinkolobwe mine was officially closed in 2004. Toponym The mine's name was taken from the long-gone nearby village of Shinkolobwe, which is the indigenous thorny fruit in the Lingala language. It is also slang for "a man who is easygoing on the surface but who becomes angry when provoked". ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marie Curie
Marie Salomea Skłodowska–Curie ( , , ; born Maria Salomea Skłodowska, ; 7 November 1867 – 4 July 1934) was a Polish and naturalized-French physicist and chemist who conducted pioneering research on radioactivity. She was the first woman to win a Nobel Prize, the first person and the only woman to win a Nobel Prize twice, and the only person to win a Nobel Prize in two scientific fields. Her husband, Pierre Curie, was a co-winner of her first Nobel Prize, making them the first-ever married couple to win the Nobel Prize and launching the Curie family legacy of five Nobel Prizes. She was, in 1906, the first woman to become a professor at the University of Paris. She was born in Warsaw, in what was then the Kingdom of Poland, part of the Russian Empire. She studied at Warsaw's clandestine Flying University and began her practical scientific training in Warsaw. In 1891, aged 24, she followed her elder sister Bronisława to study in Paris, where she earned her high ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide Mineral
The oxide mineral class includes those minerals in which the oxide anion (O2−) is bonded to one or more metal alloys. The hydroxide-bearing minerals are typically included in the oxide class. The minerals with complex anion groups such as the silicates, sulfates, carbonates and phosphates are classed separately. Simple oxides: *XO **Periclase group *** Periclase *** Manganosite **Zincite group *** Zincite *** Bromellite ***Tenorite ***Litharge * **Cuprite ** Ice * **Hematite group *** Corundum *** Hematite *** Ilmenite * **Rutile group *** Rutile *** Pyrolusite *** Cassiterite ** Baddeleyite ** Uraninite **Thorianite * **Spinel group *** Spinel ***Gahnite *** Magnetite ***Franklinite *** Chromite ** Chrysoberyl **Columbite *Hydroxide subgroup: **Brucite ** Manganite **Romanèchite **Goethite group: ***Diaspore *** Goethite Nickel–Strunz Classification -04- Oxides IMA-CNMNC proposes a new hierarchical scheme (Mills et al., 2009). This ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal. Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , row oC, column Primitive, where the cell parameters are given as a1 = a2, α = β = 90° it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the prim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha decay ( ), beta decay ( ), and gamma decay ( ), all of which involve emitting one or more particles. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by the electromagnetism and nuclear force. A fourth type of common decay is electron capture, in which an unstable nucleus captures an inner electron from one of the electron shells. The loss of that electron from the shell results in a cascade of electrons dropping down to that lower shell resulting in emission of discrete X-rays from the transitions. A common example is iodine-125 commonly used in medical settings. Radioactive decay is a stochastic (i.e. random) pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is toxic, even in small amounts, especially to children. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its amphoteric nature; lead and lead oxides react with acids and bases, and it tends to form covalent bonds. Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited to organolead compounds. Like the lighter members of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly radioactive because all isotopes of uranium are unstable; the half-lives of its naturally occurring isotopes range between 159,200 years and 4.5 billion years. The most common isotopes in natural uranium are uranium-238 (which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons). Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead, and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite. In nature, uranium is found as uranium-238 (99. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide Mineral
The oxide mineral class includes those minerals in which the oxide anion (O2−) is bonded to one or more metal alloys. The hydroxide-bearing minerals are typically included in the oxide class. The minerals with complex anion groups such as the silicates, sulfates, carbonates and phosphates are classed separately. Simple oxides: *XO **Periclase group *** Periclase *** Manganosite **Zincite group *** Zincite *** Bromellite ***Tenorite ***Litharge * **Cuprite ** Ice * **Hematite group *** Corundum *** Hematite *** Ilmenite * **Rutile group *** Rutile *** Pyrolusite *** Cassiterite ** Baddeleyite ** Uraninite **Thorianite * **Spinel group *** Spinel ***Gahnite *** Magnetite ***Franklinite *** Chromite ** Chrysoberyl **Columbite *Hydroxide subgroup: **Brucite ** Manganite **Romanèchite **Goethite group: ***Diaspore *** Goethite Nickel–Strunz Classification -04- Oxides IMA-CNMNC proposes a new hierarchical scheme (Mills et al., 2009). This ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pierre Curie
Pierre Curie ( , ; 15 May 1859 – 19 April 1906) was a French physicist, a pioneer in crystallography, magnetism, piezoelectricity, and radioactivity. In 1903, he received the Nobel Prize in Physics with his wife, Marie Curie, and Henri Becquerel, "in recognition of the extraordinary services they have rendered by their joint researches on the radiation phenomena discovered by Professor Henri Becquerel". With their win, the Curies became the first ever married couple to win the Nobel Prize, launching the Curie family legacy of five Nobel Prizes. Early life Born in Paris on 15 May 1859, Pierre Curie was the son of Eugène Curie (1827–1910), a doctor of French Catholic origin from Alsace, and Sophie-Claire Curie (née Depouilly; 1832–1897). He was educated by his father and in his early teens showed a strong aptitude for mathematics and geometry. When he was 16, he earned his Bachelor of Science in mathematics. By the age of 18, he earned his license, the equivalent of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type Locality (geology)
Type locality, also called type area, is the locality where a particular rock type, stratigraphic unit or mineral species is first identified. If the stratigraphic unit in a locality is layered, it is called a stratotype, whereas the standard of reference for unlayered rocks is the type locality. The term is similar to the term type site in archaeology or the term type specimen in biology. Examples of geological type localities Rocks and minerals * Aragonite: Molina de Aragón, Guadalajara, Spain * Autunite: Autun, France * Benmoreite: Ben More (Mull), Scotland * Blairmorite: Blairmore, Alberta, Canada * Boninite: Bonin Islands, Japan * Comendite: Comende, San Pietro Island, Sardinia * Cummingtonite: Cummington, Massachusetts * Dunite: Dun Mountain, New Zealand. * Essexite: Essex County, Massachusetts, US * Fayalite: Horta, Fayal Island, Azores, Portugal * Harzburgite: Bad Harzburg, Germany * Icelandite: Thingmuli (Þingmúli), Iceland * Ijolite: Iivaara, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |