|
Coproporphyrinogen I
Coproporphyrinogen I is an isomer of coproporphyrinogen III, a metabolic intermediate in the normal biosynthesis of heme. The compound is not normally produced by the human body; its production and accumulation causes a type of porphyria. S. Sassa and A. Kappas (2000): "Molecular aspects of the inherited porphyrias". ''Journal of Internal Medicine'', volume 247, issue 2, pages 169-178. The difference between coproporphyrinogen I and III is the arrangements of the four propionic acid, carboxyethyl ("P" groups) and the four methyl groups ("M" groups). The I isomer has the sequence MP-MP-MP-MP, whereas in the III isomer it is MP-MP-MP-PM, with the last two side chains reversed. Biosynthesys Coproporphyrinogen I is not produced in the normal porphyrin biosynthesis pathway. However, if the enzyme uroporphyrinogen-III synthase, uroporphyrinogen-III cosynthaseis missing or inactive, the compound uroporphyrinogen I is produced instead of uroporphyrinogen III. The enzyme uroporphyrinogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coproporphyrinogen III
Coproporphyrinogen III is a metabolic intermediate in the biosynthesis of many compounds that are critical for living organisms, such as hemoglobin and chlorophyll. It is a colorless solid. The compound is a porphyrinogen, a class of compounds characterized by a hexahydroporphine core with various side chains. The coproporphyrinogens have the outermost hydrogen atoms of the core replaced by four methyl groups (M) and four propionic acid Propionic acid (, from the Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula CH3CH2CO2H. It is a liq ... groups (P). In coproporphyrogen III, the order around the outer ring is MP-MP-MP-PM. For comparison, coproporphyrinogen I has them in the sequence MP-MP-MP-MP. heme. Biosynthesis and metabolism In the main Porphyrin#Synthesis, porphyrin biosynthesis pathway, coproporphyrinogen III is derived f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
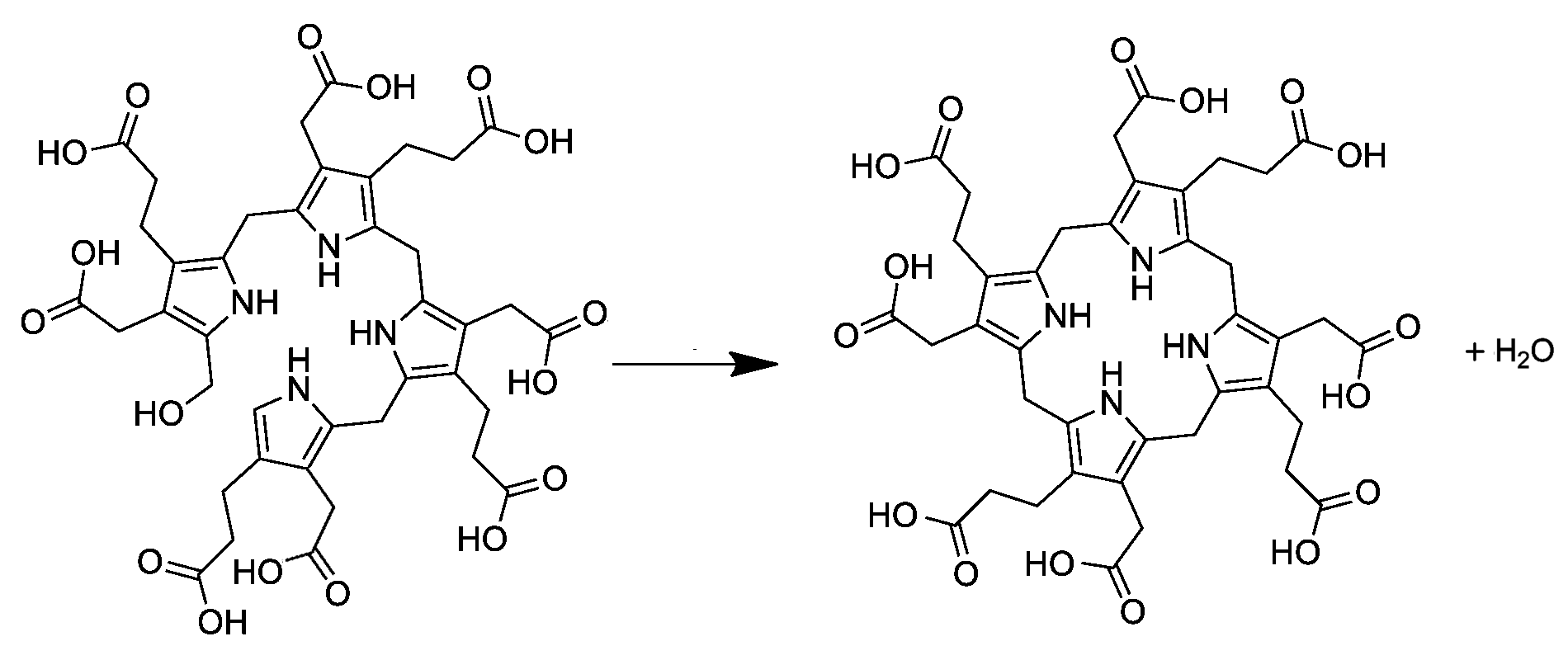
Uroporphyrinogen III
Uroporphyrinogen III is a tetrapyrrole, the first macrocyclic intermediate in the biosynthesis of heme, chlorophyll, vitamin B12, and siroheme. It is a colorless compound, like other porphyrinogens. Structure The molecular structure of uroporphyrinogen III can be described as a hexahydroporphine core, where each pyrrole ring has the hydrogen atoms on its two outermost carbons replaced by an acetic acid group (, "A") and a propionic acid group (, "P"). The groups are attached in an asymmetric way: going around the macrocycle, the order is AP-AP-AP-PA. Biosynthesis and metabolism In the general porphyrin biosynthesis pathway, uroporphyrinogen III is derived from the linear tetrapyrrole preuroporphyrinogen (a substituted hydroxymethylbilane) by the action of the enzyme uroporphyrinogen-III cosynthase. The conversion entails a reversal of the last pyrrole unit (thus swapping the acetic and propionic acid groups) and a condensation reaction that closes the macrocycle by eliminati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pathology
Pathology is the study of the causes and effects of disease or injury. The word ''pathology'' also refers to the study of disease in general, incorporating a wide range of biology research fields and medical practices. However, when used in the context of modern medical treatment, the term is often used in a narrower fashion to refer to processes and tests that fall within the contemporary medical field of "general pathology", an area which includes a number of distinct but inter-related medical specialties that diagnose disease, mostly through analysis of tissue, cell, and body fluid samples. Idiomatically, "a pathology" may also refer to the predicted or actual progression of particular diseases (as in the statement "the many different forms of cancer have diverse pathologies", in which case a more proper choice of word would be " pathophysiologies"), and the affix ''pathy'' is sometimes used to indicate a state of disease in cases of both physical ailment (as in cardiomy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytotoxicity
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa''). Cell physiology Treating cells with the cytotoxic compound can result in a variety of cell fates. The cells may undergo necrosis, in which they lose membrane integrity and die rapidly as a result of cell lysis. The cells can stop actively growing and dividing (a decrease in cell viability), or the cells can activate a genetic program of controlled cell death (apoptosis). Cells undergoing necrosis typically exhibit rapid swelling, lose membrane integrity, shut down metabolism, and release their contents into the environment. Cells that undergo rapid necrosis in vitro do not have sufficient time or energy to activate apoptotic machinery and will not express apoptotic markers. Apoptosis is characterized by well defined cytological and molecular events including a change i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol gives an elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen III Decarboxylase
Uroporphyrinogen III decarboxylase (uroporphyrinogen decarboxylase, or UROD) is an enzyme () that in humans is encoded by the ''UROD'' gene. Function Uroporphyrinogen III decarboxylase is a homodimeric enzyme () that catalyzes the fifth step in heme biosynthesis, which corresponds to the elimination of carboxyl groups from the four acetate side chains of uroporphyrinogen III to yield coproporphyrinogen III: :uroporphyrinogen III \rightleftharpoons coproporphyrinogen III + 4 CO2 Clinical significance Mutations and deficiency in this enzyme are known to cause familial porphyria cutanea tarda Porphyria cutanea tarda is the most common subtype of porphyria. The disease is named because it is a porphyria that often presents with skin manifestations later in life. The disorder results from low levels of the enzyme responsible for the fift ... and hepatoerythropoietic porphyria. At least 65 disease-causing mutations in this gene have been discovered. Mechanism At low substr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen I
Uroporphyrinogen I is an isomer of uroporphyrinogen III, a metabolic intermediate in the biosynthesis of heme. A type of porphyria is caused by production of uroporphyrinogen I instead of III. Biosynthesis and metabolism In living organisms, uroporphyrinogen I occurs as a side branch of the main porphyrin synthesis pathway. In the normal pathway, the linear tetrapyrrole precursor preuroporphyrinogen (a substituted hydroxymethylbilane) is converted by the enzyme uroporphyrinogen-III cosynthase into the cyclic uroporphyrinogen III; which is then converted to coproporphyrinogen III on the way to porphyrins like heme. Uroporphyrinogen I is instead produced spontaneously from preuroporphyrinogen when the enzyme is not present. The difference between the I and III forms is the arrangement of the four carboxyethyl groups (propionic acid, "P") and the four carboxymethyl groups (acetic acid, "A"). The non-enzymatic conversion to uroporphyrinogen I results in the sequence AP-AP-AP-AP ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolic Intermediate
Metabolic intermediates are molecules that are the precursors or metabolites of biologically significant molecules. Although these intermediates are of relatively minor direct importance to cellular function, they can play important roles in the allosteric regulation of enzymes. Clinical significance Some can be useful in measuring rates of metabolic processes (for example, 3,4-dihydroxyphenylacetic acid or 3-aminoisobutyrate). Because they can represent unnatural points of entry into natural metabolic pathways, some (such as AICA ribonucleotide) are of interest to researchers in developing new therapies. See also * Metabolism Metabolism {{chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen-III Synthase
Uroporphyrinogen III synthase () is an enzyme involved in the metabolism of the cyclic tetrapyrrole compound porphyrin. It is involved in the conversion of hydroxymethyl bilane into uroporphyrinogen III. This enzyme catalyses the inversion of the final pyrrole unit (ring D) of the linear tetrapyrrole molecule, linking it to the first pyrrole unit (ring A), thereby generating a large macrocyclic structure, uroporphyrinogen III. The enzyme folds into two alpha/beta domains connected by a beta-ladder, the active site being located between the two domains. Pathology A deficiency is associated with Gunther's disease, also known as congenital erythropoietic porphyria (CEP). This is an autosomal recessive In genetics, dominance is the phenomenon of one variant (allele) of a gene on a chromosome masking or overriding the effect of a different variant of the same gene on the other copy of the chromosome. The first variant is termed dominant and t ... inborn error of metaboli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)