|
Committed Step
In enzymology, the committed step (also known as the ''first'' committed step) is an effectively irreversible enzymatic reaction that occurs at a branch point during the biosynthesis of some molecules. As the name implies, after this step, the molecules are "committed" to the pathway and will ultimately end up in the pathway's final product. The first committed step should not be confused with the rate-determining step, which is the slowest step in a reaction or pathway. However, it is sometimes the case that the first committed step is in fact the rate-determining step as well. Regulation Metabolic pathways require tight regulation so that the proper compounds get produced in the proper amounts. Often, the first committed step is regulated by processes such as feedback inhibition and activation. Such regulation ensures that pathway intermediates do not accumulate, a situation that can be wasteful or even harmful to the cell. Examples of enzymes that catalyze the first commit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committed Step
In enzymology, the committed step (also known as the ''first'' committed step) is an effectively irreversible enzymatic reaction that occurs at a branch point during the biosynthesis of some molecules. As the name implies, after this step, the molecules are "committed" to the pathway and will ultimately end up in the pathway's final product. The first committed step should not be confused with the rate-determining step, which is the slowest step in a reaction or pathway. However, it is sometimes the case that the first committed step is in fact the rate-determining step as well. Regulation Metabolic pathways require tight regulation so that the proper compounds get produced in the proper amounts. Often, the first committed step is regulated by processes such as feedback inhibition and activation. Such regulation ensures that pathway intermediates do not accumulate, a situation that can be wasteful or even harmful to the cell. Examples of enzymes that catalyze the first commit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartate Transcarbamoylase
Aspartate carbamoyltransferase (also known as aspartate transcarbamoylase or ATCase) catalyzes the first step in the pyrimidine biosynthetic pathway (). In ''E. coli'', the enzyme is a multi- subunit protein complex composed of 12 subunits (300 kDa in total). The composition of the subunits is C6R6, forming 2 trimers of catalytic subunits (34 kDa) and 3 dimers of regulatory subunits (17 kDa). The particular arrangement of catalytic and regulatory subunits in this enzyme affords the complex with strongly allosteric behaviour with respect to its substrates. The enzyme is an archetypal example of allosteric modulation of fine control of metabolic enzyme reactions. ATCase does not follow Michaelis–Menten kinetics. Instead, it lies between its low-activity, low-affinity "tense" and its high-activity, high-affinity "relaxed" states. The binding of substrate to the catalytic subunits results in an equilibrium shift towards the R state, whereas binding of CTP to the regulatory subun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metazoan
Animals are multicellular, eukaryotic organisms in the biological kingdom Animalia. With few exceptions, animals Heterotroph, consume organic material, Cellular respiration#Aerobic respiration, breathe oxygen, are Motility, able to move, can Sexual reproduction, reproduce sexually, and go through an ontogenetic stage in which their body consists of a hollow sphere of Cell (biology), cells, the blastula, during Embryogenesis, embryonic development. Over 1.5 million Extant taxon, living animal species have been Species description, described—of which around 1 million are Insecta, insects—but it has been estimated there are over 7 million animal species in total. Animals range in length from to . They have Ecology, complex interactions with each other and their environments, forming intricate food webs. The scientific study of animals is known as zoology. Most living animal species are in Bilateria, a clade whose members have a Symmetry in biology#Bilateral symmetry, bilat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentose Phosphate Pathway
The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt and the HMP Shunt) is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses (5-carbon sugars) as well as ribose 5-phosphate, a precursor for the synthesis of nucleotides. While the pentose phosphate pathway does involve oxidation of glucose, its primary role is anabolic rather than catabolic. The pathway is especially important in red blood cells (erythrocytes). There are two distinct phases in the pathway. The first is the oxidative phase, in which NADPH is generated, and the second is the non-oxidative synthesis of 5-carbon sugars. For most organisms, the pentose phosphate pathway takes place in the cytosol; in plants, most steps take place in plastids. Like glycolysis, the pentose phosphate pathway appears to have a very ancient evolutionary origin. The reactions of this pathway are mostly enzyme-catalyzed in modern cells, however, they also occur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NADPH
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). It is used by all forms of cellular life. NADPH is the reduced form of NADP. NADP differs from NAD by the presence of an additional phosphate group on the 2' position of the ribose ring that carries the adenine moiety. This extra phosphate is added by NAD+ kinase and removed by NADP+ phosphatase. Biosynthesis NADP In general, NADP+ is synthesized before NADPH is. Such a reaction usually starts with NAD+ from either the de-novo or the salvage pathway, with NAD+ kinase adding the extra phosphate group. ADP-ribosyl cyclase allows for synthesis from nicotinamide in the salvage pathway, and NADP+ phosphatase can convert NADPH back to NADH to maintain a balance. Some forms of the NAD+ kinas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
6-Phosphogluconolactone
6-Phosphogluconolactone is an intermediate in the pentose phosphate pathway (PPP). In the PPP pathway, it is produced from glucose-6-phosphate by glucose-6-phosphate dehydrogenase. It is then converted to 6-Phosphogluconic acid by 6-phosphogluconolactonase. See also * Lactone Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring. Lactones are formed by intramolecular esterification of the co ... Organophosphates Delta-lactones {{biochem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose-6-phosphate Dehydrogenase
Glucose-6-phosphate dehydrogenase (G6PD or G6PDH) () is a cytosolic enzyme that catalyzes the chemical reaction : D-glucose 6-phosphate + NADP+ + H2O 6-phospho-D-glucono-1,5-lactone + NADPH + H+ This enzyme participates in the pentose phosphate pathway (see image), a metabolic pathway that supplies reducing energy to cells (such as erythrocytes) by maintaining the level of the co-enzyme nicotinamide adenine dinucleotide phosphate (NADPH). The NADPH in turn maintains the level of glutathione in these cells that helps protect the red blood cells against oxidative damage from compounds like hydrogen peroxide. Of greater quantitative importance is the production of NADPH for tissues involved in biosynthesis of fatty acids or isoprenoids, such as the liver, mammary glands, adipose tissue, and the adrenal glands. G6PD reduces NADP+ to NADPH while oxidizing glucose-6-phosphate. Glucose-6-phosphate dehydrogenase is also an enzyme in the Entner–Doudoroff pathway, a type of glycolysis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatty Acid Synthesis
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is converted into fatty acids is derived from carbohydrates via the glycolytic pathway. The glycolytic pathway also provides the glycerol with which three fatty acids can combine (by means of ester bonds) to form triglycerides (also known as "triacylglycerols" – to distinguish them from fatty "acids" – or simply as "fat"), the final product of the lipogenic process. When only two fatty acids combine with glycerol and the third alcohol group is phosphorylated with a group such as phosphatidylcholine, a phospholipid is formed. Phospholipids form the bulk of the lipid bilayers that make up cell membranes and surrounds the organelles within the cells (such as the cell nucleus, mitochondria, endoplasmic reticulum, Golgi apparatus, etc.). Straight ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malonyl-CoA
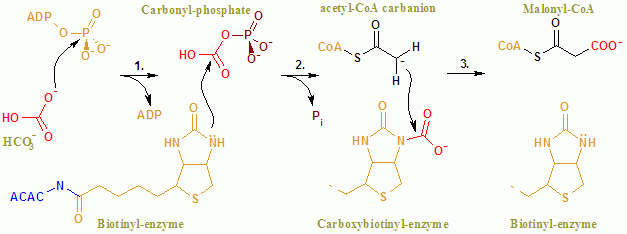
Malonyl-CoA is a coenzyme A derivative of malonic acid. Functions It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis. Fatty acid biosynthesis Malonyl-CoA provides 2-carbon units to fatty acids and commits them to fatty acid chain synthesis. Malonyl-CoA is formed by carboxylating acetyl-CoA using the enzyme acetyl-CoA carboxylase. One molecule of acetyl-CoA joins with a molecule of bicarbonate,Nelson D, Cox M (2008) ''Lehninger principles of biochemistry''. 5th Ed: p. 806 requiring energy rendered from ATP. Malonyl-CoA is utilised in fatty acid biosynthesis by the enzyme malonyl coenzyme A:acyl carrier protein transacylase (MCAT). MCAT serves to transfer malonate from malonyl-CoA to the terminal thiol of ''holo''-acyl carrier protein (ACP). Polyketide biosynthesis MCAT is also involved in bacterial polyketide biosynthesis. The enzyme MCAT together with an acyl carrier protein (ACP), and a polyketide synthase (PKS) and chain-length f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylation
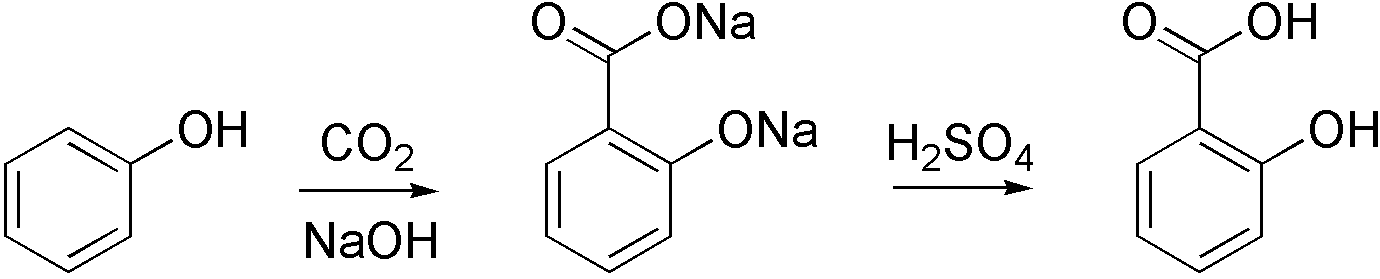
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylation, especially when applied to the reaction of carbanionic reagents with CO2. More generally, carbonation usually describes the production of carbonates. Organic chemistry Carboxylation is a standard conversion in organic chemistry. Specifically carbonation (i.e. carboxylation) of Grignard reagents and organolithium compounds is a classic way to convert organic halides into carboxylic acids. Sodium salicylate, precursor to aspirin, is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (390 K) – a method known as the Kolbe-Schmitt reaction. Acidification of the resulting salicylate salt gives salicylic acid. : Many detailed procedures are des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl-CoA Carboxylase
Acetyl-CoA carboxylase (ACC) is a biotin-dependent enzyme () that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA through its two catalytic activities, biotin carboxylase (BC) and carboxyltransferase (CT). ACC is a multi-subunit enzyme in most prokaryotes and in the chloroplasts of most plants and algae, whereas it is a large, multi-domain enzyme in the cytoplasm of most eukaryotes. The most important function of ACC is to provide the malonyl-CoA substrate for the biosynthesis of fatty acids. The activity of ACC can be controlled at the transcriptional level as well as by small molecule modulators and covalent modification. The human genome contains the genes for two different ACCs—'' ACACA'' and ''ACACB''. Structure Prokaryotes and plants have multi-subunit ACCs composed of several polypeptides. Biotin carboxylase (BC) activity, biotin carboxyl carrier protein (BCCP), and carboxyl transferase (CT) activity are each contained on a different s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citric Acid Cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism and may have originated abiogenically. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized. The name of this metabolic pathway is derived from the citric acid (a tricarboxy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |