|
Calcium Cycle
The calcium cycle is a transfer of calcium between dissolved and solid phases. There is a continuous supply of calcium ions into waterways from rocks, organisms, and soils. Calcium ions are consumed and removed from aqueous environments as they react to form insoluble structures such as calcium carbonate and calcium silicate, which can deposit to form sediments or the exoskeletons of organisms. Calcium ions can also be utilized biologically, as calcium is essential to biological functions such as the production of bones and teeth or cellular function. The calcium cycle is a common thread between terrestrial, marine, geological, and biological processes. Calcium moves through these different media as it cycles throughout the Earth. The marine calcium cycle is affected by changing atmospheric carbon dioxide due to ocean acidification. Calcium weathering and inputs to seawater Calcium is stored in geologic reservoirs, most commonly in the form of calcium carbonate or as calcium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be " miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Physics'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' "lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
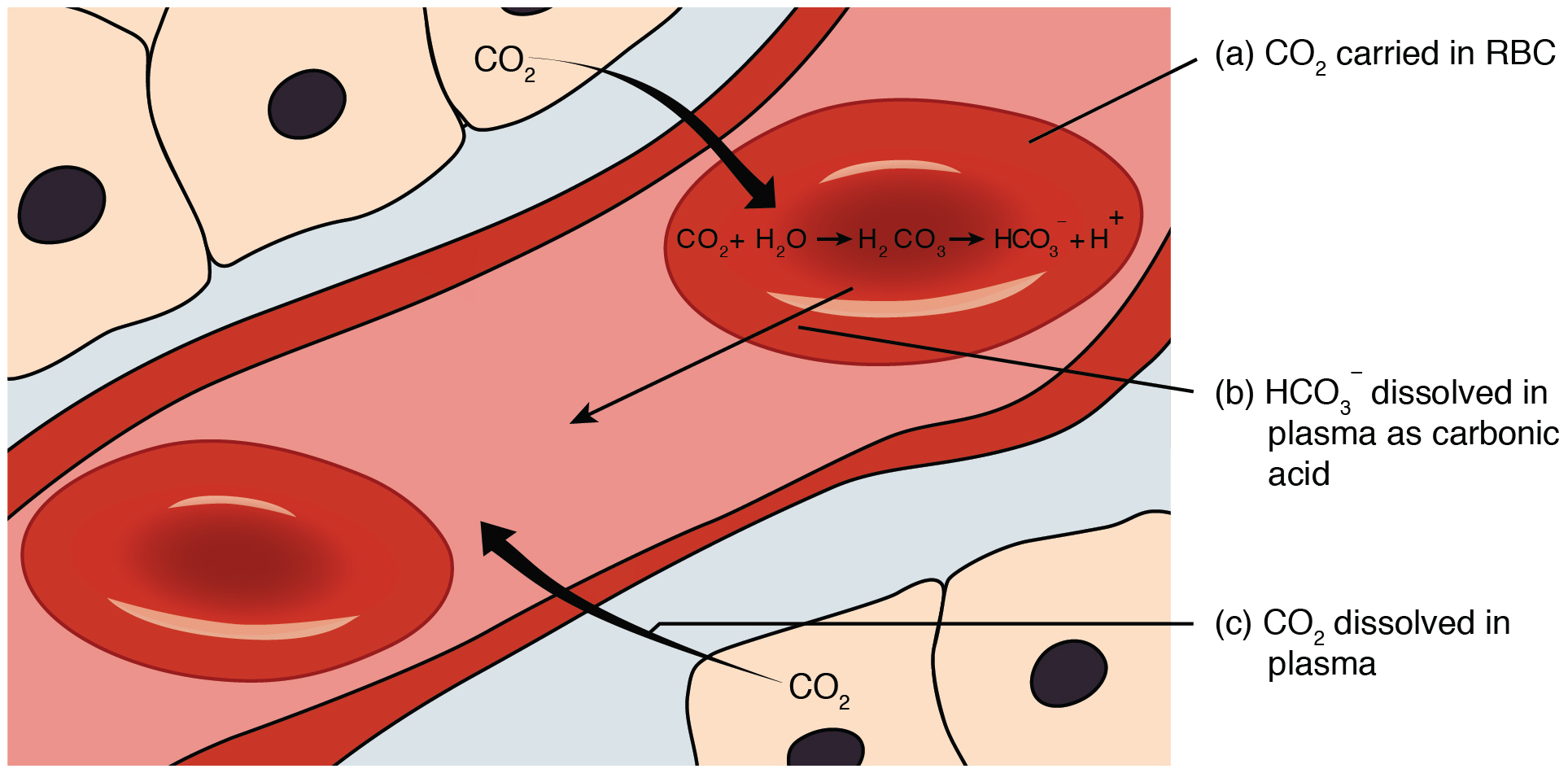
Bicarbonate Buffer System
The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid (H2CO3), bicarbonate ion (HCO), and carbon dioxide (CO2) in order to maintain pH in the blood and duodenum, among other tissues, to support proper metabolic function. Catalyzed by carbonic anhydrase, carbon dioxide (CO2) reacts with water (H2O) to form carbonic acid (H2CO3), which in turn rapidly dissociates to form a bicarbonate ion (HCO ) and a hydrogen ion (H+) as shown in the following reaction: \rm CO_2 + H_2O \rightleftarrows H_2CO_3 \rightleftarrows HCO_3^- + H^+ As with any buffer system, the pH is balanced by the presence of both a weak acid (for example, H2CO3) and its conjugate base (for example, HCO) so that any excess acid or base introduced to the system is neutralized. Failure of this system to function properly results in acid-base imbalance, such as acidemia (pH 7.45) in the blood. In systemic acid–base balance In tissue, cellular respiration produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms when these minerals precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes, such as the accumulation of corals and shells in the sea, have likely been more important for the last 540 million years. Limestone often contains fossils which provide scientists with information on ancient environments and on the evolution of life. About 20% to 25% of sedimentary rock is carbonate rock, and most of this is limestone. The remaining carbonate rock is mostly dolomite, a closely related rock, which contains a high percentage of the mineral dolomite, . ''Magnesian limestone'' is an obsolete and poorly-defined term used variously for dolomite, for limes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aragonite
Aragonite is a carbonate mineral, one of the three most common naturally occurring crystal forms of calcium carbonate, (the other forms being the minerals calcite and vaterite). It is formed by biological and physical processes, including precipitation from marine and freshwater environments. The crystal lattice of aragonite differs from that of calcite, resulting in a different crystal shape, an orthorhombic crystal system with acicular crystal. Repeated twinning results in pseudo-hexagonal forms. Aragonite may be columnar or fibrous, occasionally in branching helictitic forms called ''flos-ferri'' ("flowers of iron") from their association with the ores at the Carinthian iron mines. Occurrence The type location for aragonite is Molina de Aragón in the Province of Guadalajara in Castilla-La Mancha, Spain, for which it was named in 1797. Aragonite is found in this locality as cyclic twins inside gypsum and marls of the Keuper facies of the Triassic. This type of arago ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula . Bicarbonate serves a crucial biochemical role in the physiological pH buffering system. The term "bicarbonate" was coined in 1814 by the English chemist William Hyde Wollaston. The name lives on as a trivial name. Chemical properties The bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula and a molecular mass of 61.01 daltons; it consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a hydrogen atom attached to one of the oxygens. It is isoelectronic with nitric acid . The bicarbonate ion carries a negative one formal charge and is an amphiprotic species which has both acidic and basic properties. It is both the conjugate base of carbonic acid ; and the conjugate acid of , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mollusca
Mollusca is the second-largest phylum of invertebrate animals after the Arthropoda, the members of which are known as molluscs or mollusks (). Around 85,000 extant species of molluscs are recognized. The number of fossil species is estimated between 60,000 and 100,000 additional species. The proportion of undescribed species is very high. Many taxa remain poorly studied. Molluscs are the largest marine phylum, comprising about 23% of all the named marine organisms. Numerous molluscs also live in freshwater and terrestrial habitats. They are highly diverse, not just in size and anatomical structure, but also in behaviour and habitat. The phylum is typically divided into 7 or 8 taxonomic classes, of which two are entirely extinct. Cephalopod molluscs, such as squid, cuttlefish, and octopuses, are among the most neurologically advanced of all invertebrates—and either the giant squid or the colossal squid is the largest known invertebrate species. The gastropod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pteropoda
Pteropoda (common name pteropods, from the Greek meaning "wing-foot") are specialized free-swimming pelagic sea snails and sea slugs, marine opisthobranch gastropods. Most live in the top 10 m of the ocean and are less than 1 cm long. The monophyly of Pteropoda is the subject of a lengthy debate; they have even been considered as paraphyletic with respect to cephalopods. Current consensus, guided by molecular studies, leans towards interpreting the group as monophyletic. Pteropoda encompasses the two clades Thecosomata, the sea butterflies, and Gymnosomata, the sea angels. The Thecosomata ( "case-body") have a shell, while the Gymnosomata ("naked body") do not. The two clades may or may not be sister taxa; if not, their similarity (in that they are both pelagic, small, and transparent, and both groups swim using wing-like flaps (parapodia) which protrude from their bodies) may reflect adaptation to their particular lifestyle. Taxonomy The group Pteropoda was established by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coral
Corals are marine invertebrates within the class Anthozoa of the phylum Cnidaria. They typically form compact colonies of many identical individual polyps. Coral species include the important reef builders that inhabit tropical oceans and secrete calcium carbonate to form a hard skeleton. A coral "group" is a colony of very many genetically identical polyps. Each polyp is a sac-like animal typically only a few millimeters in diameter and a few centimeters in height. A set of tentacles surround a central mouth opening. Each polyp excretes an exoskeleton near the base. Over many generations, the colony thus creates a skeleton characteristic of the species which can measure up to several meters in size. Individual colonies grow by asexual reproduction of polyps. Corals also breed sexually by spawning: polyps of the same species release gametes simultaneously overnight, often around a full moon. Fertilized eggs form planulae, a mobile early form of the coral polyp which, when m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
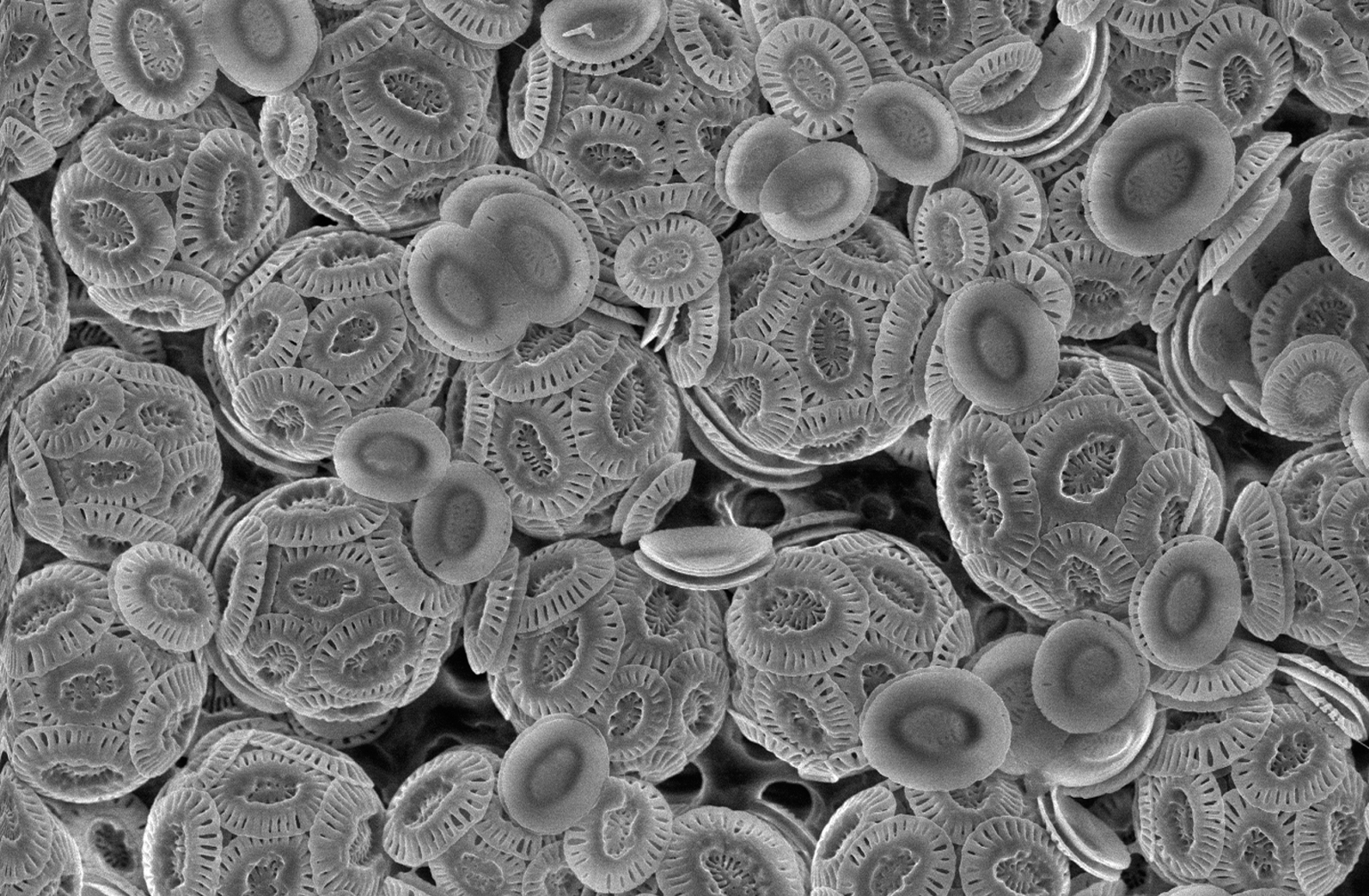
Coccolithophore
Coccolithophores, or coccolithophorids, are single celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingdom Protista, according to Robert Whittaker's Five kingdom classification, or clade Hacrobia, according to a newer biological classification system. Within the Hacrobia, the coccolithophores are in the phylum or division Haptophyta, class Prymnesiophyceae (or Coccolithophyceae). Coccolithophores are almost exclusively marine, are photosynthetic, and exist in large numbers throughout the sunlight zone of the ocean. Coccolithophores are the most productive calcifying organisms on the planet, covering themselves with a calcium carbonate shell called a ''coccosphere''. However, the reasons they calcify remains elusive. One key function may be that the coccosphere offers protection against microzooplankton predation, which is one of the ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dolomitization
Dolomitization is a geological process by which the carbonate mineral dolomite is formed when magnesium ions replace calcium ions in another carbonate mineral, calcite. It is common for this mineral alteration into dolomite to take place due to evaporation of water in the sabkha area. Dolomitization involves substantial amount of recrystallization. This process is described by the stoichiometric equation: :2 CaCO3(calcite) + Mg2+ ↔ CaMg(CO3)2(dolomite) + Ca2+ Dolomitization depends on specific conditions which include low Ca:Mg ratio in solution, reactant surface area, the mineralogy of the reactant, high temperatures which represents the thermodynamic stability of the system, and the presence of kinetic inhibitors such as sulfate. If the kinetic inhibitors and high temperatures are compatible, then dolomitization can take place in saline environments above thermodynamic and kinetic saturation with respect to dolomite. This type of environment includes, freshwater and seawate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywall. Alabaster, a fine-grained white or lightly tinted variety of gypsum, has been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Gypsum also crystallizes as translucent crystals of selenite. It forms as an evaporite mineral and as a hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on scratch hardness comparison. Etymology and history The word ''gypsum'' is derived from the Greek word (), "plaster". Because the quarries of the Montmartre district of Paris have long furnished burnt gypsum (calcined gypsum) used for various purposes, this dehydrated gypsum became known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.jpg)