|
Comproportionation
Comproportionation or symproportionation is a chemical reaction where two reactants containing the same element but with different oxidation numbers, form a compound having an intermediate oxidation number. It is the opposite of disproportionation.Shriver, D. F.; Atkins, P. W.; Overton, T. L.; Rourke, J. P.; Weller, M. T.; Armstrong, F. A. (2006). “Inorganic Chemistry” W. H. Freeman, New York. . Frost diagrams In electrochemistry, the tendency of two redox species to disproportionate, or comproportionate, can be determined by examining their Frost diagram. It is a graphical plot of as a function of the oxidation number for the different redox species of a given element. The Gibbs free energy Δ''G''° is related to the reduction potential ''E''° by the formula: or , where ''n'' is the number of transferred electrons, and ''F'' is the Faraday constant ). If the value of for a species is lower than the line joining two adjacent, or more generally, neighboring speci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
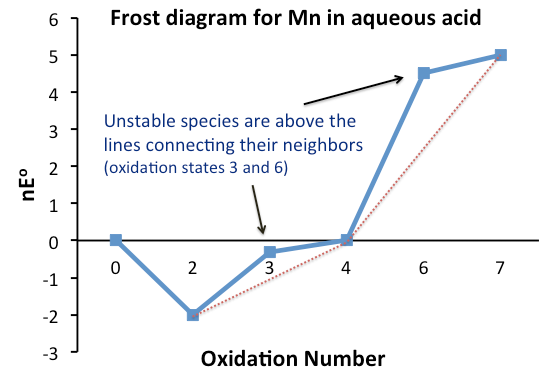
Frost Diagram
A Frost diagram or Frost–Ebsworth diagram is a type of graph used by inorganic chemists in electrochemistry to illustrate the relative stability of a number of different oxidation states of a particular substance. The graph illustrates the free energy vs oxidation state of a chemical species. This effect is dependent on pH, so this parameter also must be included. The free energy is determined by the oxidation–reduction half-reactions. The Frost diagram allows easier comprehension of these reduction potentials than the earlier-designed Latimer diagram, because the “lack of additivity of potentials” was confusing. The free energy Δ''G''° is related to the standard electrode potential ''E''° shown in the graph by the formula: or , where ''n'' is the number of transferred electrons, and ''F'' is the Faraday constant . The Frost diagram is named after , who originally invented it as a way to "show both free energy and oxidation potential data conveniently" in a 1951 pap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called ''comproportionation'', also known as ''symproportionation''. More generally, the term can be applied to any desymmetrizing reaction where two molecules of one type react to give one each of two different types: : This expanded definition is not limited to redox reactions, but also includes some molecular autoionization reactions, such as the self-ionization of water. In contrast, some authors use the term ''redistribution'' to refer to reactions of this type (in either direction) when only ligand exchange but no redox is involved and distinguish such processes from disproportionation and comproportionati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Chloride
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions. Production Hydrated forms of ferrous chloride are generated by treatment of wastes from steel production with hydrochloric acid. Such solutions are designated "spent acid," or "pickle liquor" especially when the hydrochloric acid is not completely consumed: :Fe + 2 HCl → FeCl2 + H2 The production of ferric chloride involves the use of ferrous chloride. Ferrous chloride is also a byproduct from the production of titanium, since some titanium ores contain iron.Egon Wildermuth, Hans Stark, Gabriele Friedrich, Franz Ludw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(III) Chloride
Iron(III) chloride describes the inorganic compounds with the formula (H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms, which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water cleaner and as an etchant for metals. Electronic and optical properties All forms of ferric chloride are paramagnetic, owing to the presence of unpaired electrons residing in 3d orbitals. Although Fe(III) chloride can be octahedral or tetrahedral (or both, see structure section), all of these forms have five unpaired electrons, one per d-orbital. The high spin d5 electronic configuration requires that d-d electronic transitions are spin forbidden, in addition to violating the Laporte rule. This double forbidden-ness results in its solutions being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anammox
Anammox, an abbreviation for "anaerobic ammonium oxidation", is a globally important microbial process of the nitrogen cycle that takes place in many natural environments. The bacteria mediating this process were identified in 1999, and were a great surprise for the scientific community. In the anammox reaction, nitrite and ammonium ions are converted directly into diatomic nitrogen and water. The bacteria that perform the anammox process are genera that belong to the bacterial phylum Planctomycetota. The anammox bacteria all possess one anammoxosome, a lipid bilayer membrane-bound compartment inside the cytoplasm in which the anammox process takes place. The anammoxosome membranes are rich in ladderane lipids; the presence of these lipids is so far unique in biology. "Anammox" is also the trademarked name for an anammox-based ammonium removal technology developedJetten Michael Silvester Maria, Van Loosdrecht Marinus Corneli; Technische Universiteit Delftpatent WO9807664/ref> b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with the chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most common on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead Dioxide
Lead(IV) oxide, commonly known as lead dioxide, is an inorganic compound with the chemical formula . It is an oxide where lead is in an oxidation state of +4. It is a dark-brown solid which is insoluble in water. It exists in two crystalline forms. It has several important applications in electrochemistry, in particular as the positive plate of lead acid batteries. Properties Physical Lead dioxide has two major polymorphs, alpha and beta, which occur naturally as rare minerals scrutinyite and plattnerite, respectively. Whereas the beta form had been identified in 1845, α- was first identified in 1946 and found as a naturally occurring mineral 1988. The alpha form has orthorhombic symmetry, space group Pbcn (No. 60), Pearson symbol ''oP''12, lattice constants ''a'' = 0.497 nm, ''b'' = 0.596 nm, ''c'' = 0.544 nm, ''Z'' = 4 (four formula units per unit cell). The lead atoms are six-coordinate. The symmetry of the beta form is tetragonal, space group P42/mnm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition metal with a multifaceted array of industrial alloy uses, particularly in stainless steels. It improves strength, workability, and resistance to wear. Manganese oxide is used as an oxidising agent, as a rubber additive, and in glass making, fertilisers, and ceramics. Manganese sulfate can be used as a fungicide. Manganese is also an essential human dietary element, important in macronutrient metabolism, bone formation, and free radical defense systems. It is a critical component in dozens of proteins and enzymes. It is found mostly in the bones, but also the liver, kidneys, and brain. In the human brain, the manganese is bound to manganese metalloproteins, most notably glutamine synthetase in astrocytes. Manganese is commonly found in labo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropes Of Sulfur
The element sulfur exists as many allotropes. In number of allotropes, sulfur is second only to Allotropes of carbon, carbon.#Greenwood, Greenwood, 652 In addition to the allotropes, each allotrope often exists in Polymorphism (materials science), polymorphs (different crystal structures of the same covalently bonded Sn molecules) delineated by Greek prefixes (α, β, etc.). Furthermore, because elemental sulfur has been an item of commerce for centuries, its various forms are given traditional names. Early workers identified some forms that have later proved to be single or mixtures of allotropes. Some forms have been named for their appearance, e.g. "mother of pearl sulfur", or alternatively named for a chemist who was pre-eminent in identifying them, e.g. "Muthmann's sulfur I" or "Engel's sulfur".#Steudel, Steudel, 17 The most commonly encountered form of sulfur is the orthorhombic polymorph of , which adopts a puckered ring – or "crown" – structure. Two other polymorphs a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most abundant element in the Earth's crust, being mainly deposited by meteorites in its metallic state. Extracting usable metal from iron ores requires kilns or furnaces capable of reaching , about 500 °C (900 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BC and the use of iron tools and weapons began to displace copper alloys – in some regions, only around 1200 BC. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, due to their mechan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronically conducting phase (typically an external electrical circuit, but not necessarily, as in Electroless nickel-phosphorus plating, electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic chemical species, species in a Solution (chemistry), solution). When a chemical reaction is driven by an electrical Voltage, potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via the aforementioned electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |