|
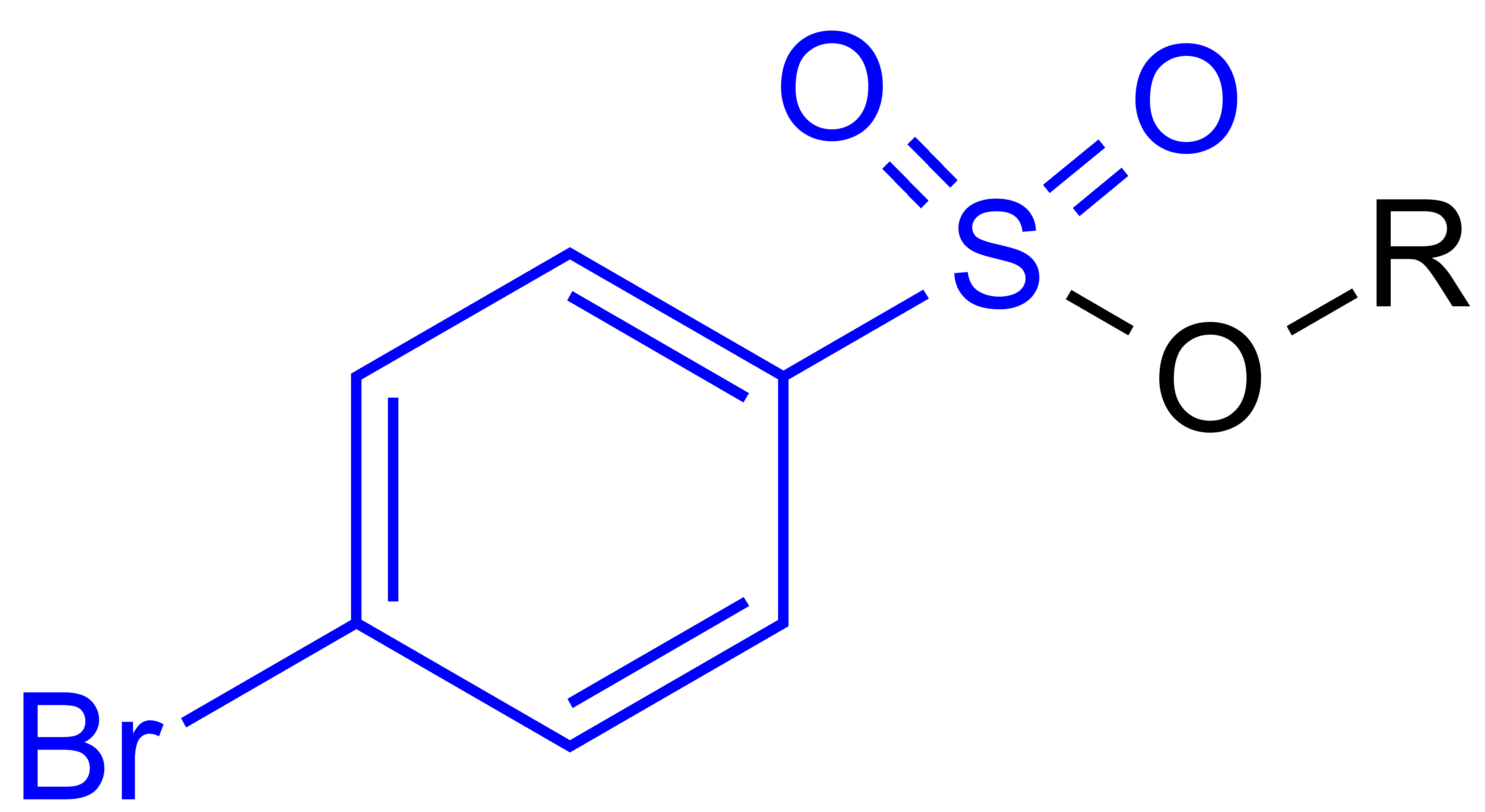
Brosylate
In organic chemistry, brosyl (or ''para''-bromophenylsulfonyl) group is a functional group with the chemical formula BrC6H4SO2. This group is usually introduced using the compound brosyl chloride, BrC6H4SO2Cl, which forms sulfonyl esters and amides of ''p''-bromophenylsulfonic acid. The term brosylate refers to the anion of ''p''-bromophenylsulfonic acid (BrC6H4SO3−). See also * Tosyl group * Tosylic acid * Triflic acid * Sulfonyl group Sulfonyl groups Functional groups References {{organic-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brosyl Group General Formulae
In organic chemistry, brosyl (or ''para''-bromophenylsulfonyl) group is a functional group with the chemical formula BrC6H4SO2. This group is usually introduced using the compound brosyl chloride, BrC6H4SO2Cl, which forms sulfonyl esters and amides of ''p''-bromophenylsulfonic acid. The term brosylate refers to the anion of ''p''-bromophenylsulfonic acid (BrC6H4SO3−). See also * Tosyl group * Tosylic acid * Triflic acid Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for este ... * Sulfonyl group Sulfonyl groups Functional groups References {{organic-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brosyl Chloride
In organic chemistry, brosyl (or ''para''-bromophenylsulfonyl) group is a functional group with the chemical formula BrC6H4SO2. This group is usually introduced using the compound brosyl chloride, BrC6H4SO2Cl, which forms sulfonyl esters and amides of ''p''-bromophenylsulfonic acid. The term brosylate refers to the anion of ''p''-bromophenylsulfonic acid (BrC6H4SO3−). See also * Tosyl group * Tosylic acid * Triflic acid Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for este ... * Sulfonyl group Sulfonyl groups Functional groups References {{organic-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tosyl Group
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula –. It consists of a tolyl group, –, joined to a sulfonyl group, ––, with the open valence on sulfur. This group is usually derived from the compound tosyl chloride, (abbreviated TsCl), which forms esters and amides of toluenesulfonic acid, (abbreviated TsOH). The para orientation illustrated (''p''-toluenesulfonyl) is most common, and by convention ''tosyl'' without a prefix refers to the ''p''-toluenesulfonyl group. The toluenesulfonate (or tosylate) group refers to the – (TsO–) group, with an additional oxygen attached to sulfur and open valence on an oxygen. In a chemical name, the term ''tosylate'' may either refer to the salts containing the anion of ''p''-toluenesulfonic acid, (M = alkali metal, , , etc), or it may refer to esters of ''p''-toluenesulfonic acid, TsOR (R = organyl group). Applications For SN2 reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonyl Ester
In organosulfur chemistry, a sulfonate is a salt or ester of a sulfonic acid. It contains the functional group , where R is an organic group. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non-oxidizing, and colorless. Many useful compounds and even some biochemicals feature sulfonates. Sulfonate salts Anions with the general formula are called sulfonates. They are the conjugate bases of sulfonic acids with formula . As sulfonic acids tend to be strong acids, the corresponding sulfonates are weak bases. Due to the stability of sulfonate anions, the cations of sulfonate salts such as scandium triflate have application as Lewis acids. A classic preparation of sulfonates is the Strecker sulfite alkylation, in which an alkali sulfite salt displaces a halide, typically in the presence of an iodine catalyst: :RX + M2SO3 -> RSO3M + MX An alternative is the condensation of a sulfonyl halide with an alcohol in pyridine: :R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tosylic Acid
''p''-Toluenesulfonic acid (PTSA or ''p''TsOH) or tosylic acid (TsOH) is an organic compound with the formula CH3 C6H4 SO3H. It is a white extremely hygroscopic solid that is soluble in water, alcohols, and other polar organic solvents. The CH3C6H4SO2 group is known as the tosyl group and is often abbreviated as Ts or Tos. Most often, TsOH refers to the monohydrate, TsOH.H2O. As with other aryl sulfonic acids, TsOH is a strong organic acid. It is about one million times stronger than benzoic acid. It is one of the few strong acids that is solid and therefore is conveniently weighed and stored. Preparation and uses TsOH is prepared on an industrial scale by the sulfonation of toluene. Common impurities include benzenesulfonic acid and sulfuric acid. TsOH monohydrate contains an amount of water. To estimate the total moisture present as impurity, the Karl Fischer method is used. Impurities can be removed by recrystallization from its concentrated aqueous solution followed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triflic Acid
Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for esterification. It is a hygroscopic, colorless, slightly viscous liquid and is soluble in polar solvents. Synthesis Trifluoromethanesulfonic acid is produced industrially by electrochemical fluorination (ECF) of methanesulfonic acid: : CH3SO3H + 4 HF ->CF3SO2F + H2O + 3 H2 The resulting CF3SO2F is hydrolyzed, and the resulting triflate salt is reprotonated. Alternatively, trifluoromethanesulfonic acid arises by oxidation of trifluoromethylsulfenyl chloride: :CF3SCl + 2 Cl2 + 3 H2O -> CF3SO3H + 5 HCl Triflic acid is purified by distillation from triflic anhydride. Historical Trifluoromethanesulfonic acid was first synthesized in 1954 by Robert Haszeldine and Kidd by the following reaction: : Reactions As an acid In the laboratory, triflic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonyl Group
In organosulfur chemistry, a sulfonyl group can refer either to a functional group found primarily in sulfones, or to a substituent obtained from a sulfonic acid by the removal of the hydroxyl group, similarly to acyl groups. Sulfonyl groups can be written as having the general formula , where there are two double bonds between the sulfur and oxygen. Sulfonyl groups can be reduced to the sulfide with DIBALH. Lithium aluminium hydride () reduces some but not all sulfones to sulfides. In inorganic chemistry, when the group is not connected to any carbon atoms, it is referred to as sulfuryl. Examples of sulfonyl group substituents The names of sulfonyl groups typically end in -syl, such as: : See also * Sulfonyl halide * Sulfonamide * Sulfonate In organosulfur chemistry, a sulfonate is a salt or ester of a sulfonic acid. It contains the functional group , where R is an organic group. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonyl Groups
In organosulfur chemistry, a sulfonyl group can refer either to a functional group found primarily in sulfones, or to a substituent obtained from a sulfonic acid by the removal of the hydroxyl group, similarly to acyl groups. Sulfonyl groups can be written as having the general formula , where there are two double bonds between the sulfur and oxygen. Sulfonyl groups can be reduced to the sulfide with DIBALH. Lithium aluminium hydride () reduces some but not all sulfones to sulfides. In inorganic chemistry, when the group is not connected to any carbon atoms, it is referred to as sulfuryl. Examples of sulfonyl group substituents The names of sulfonyl groups typically end in -syl, such as: : See also * Sulfonyl halide * Sulfonamide * Sulfonate In organosulfur chemistry, a sulfonate is a salt or ester of a sulfonic acid. It contains the functional group , where R is an organic group. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are general ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |