|
Boronium
In chemistry, a boranylium ion is an inorganic compound, inorganic cation with the chemical formula , where R represents a non-specific substituent. Being electron-deficient, boranylium ions form adducts with Lewis bases. Boranylium ions have historical names that depend on the number of coordinated ligands: *: borinium *: borenium *: boronium Borenium ions A borenium ion is an inorganic compound, inorganic cation with the chemical formula . In this class of molecules, the electron-deficient boron center has two valence electrons involved in sigma bonding with two ligands, while the third ligand is a two-electron donor such that the overall charge of the complex is +1. Depending on the nature of the ligands around the central boron, this positive charge can be localized on the boron center or delocalized across the entire molecule. Borenium ions can be made in a number of different ways and are of interest for applications in organic synthesis and catalysis. Synthesis Synthetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
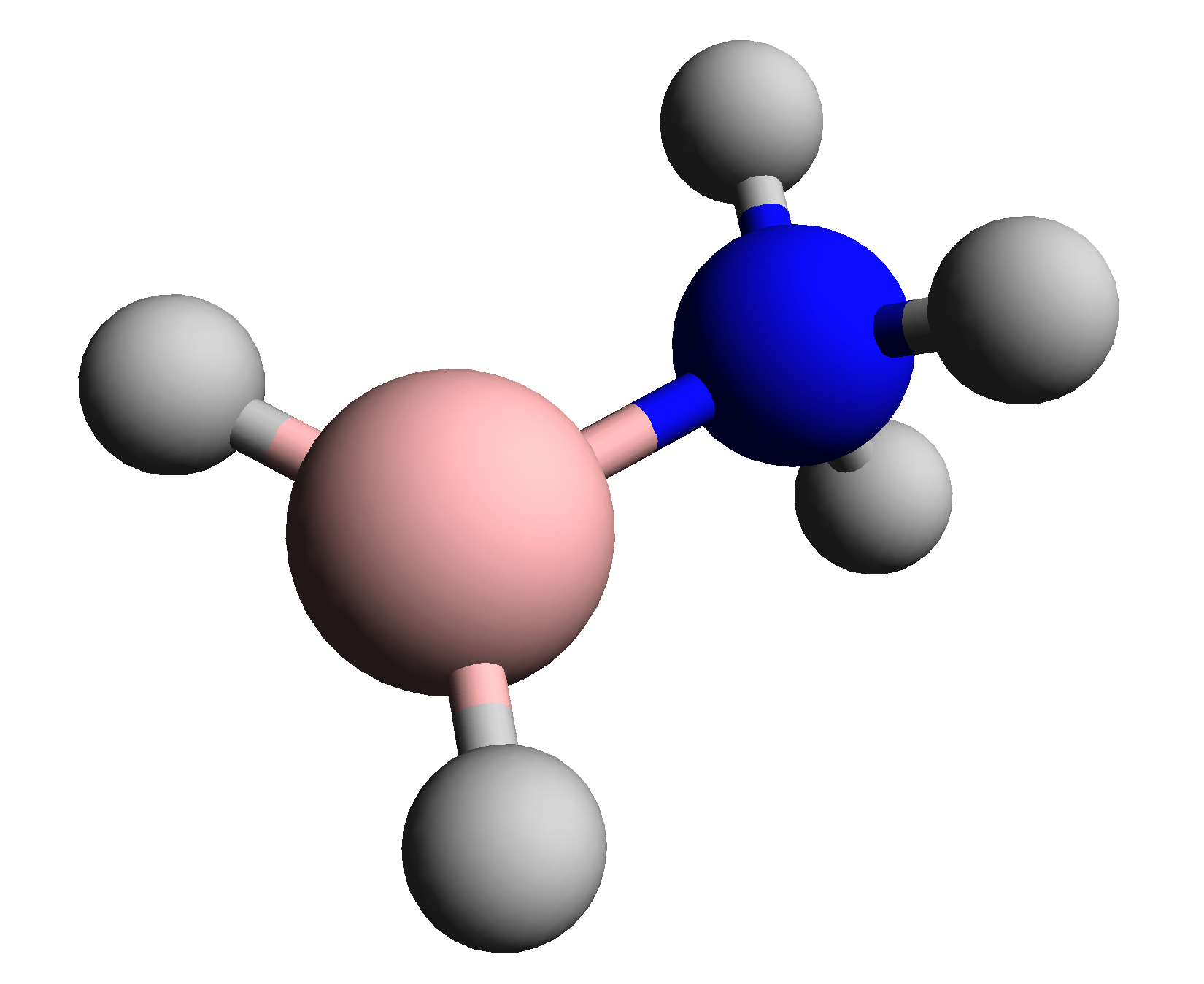
Bh2nh3
BH, Bh or bh may refer to: Medicine * Bernard-Horner syndrome, a combination of symptoms that arises when a group of nerves known as the sympathetic trunk is damaged * Borderline hypertensive, an American medical classification for cases where a person's blood pressure is elevated above normal, but not to the level considered hypertension * Bronchial hyperresponsiveness, a state characterised by easily triggered bronchospasm * Bundle of His, collection of heart muscle cells specialized for electrical conduction Science and technology * BH register, in computer architectures * Bohrium, symbol Bh, a chemical element * Boron monohydride, chemical formula BH, a chemical compound * Black hole Places * BH postcode area, a region in southern England served by Bournemouth postal sorting office * Bahrain (ISO 3166-1 country code BH) ** .bh, the Internet country code top-level domain for Bahrain * Belize's WMO and obsolete NATO country code digram * Belo Horizonte, the capital of Min ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protic Attack Mech
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a labile is called a protic solvent. The molecules of such solvents readily donate protons () to solutes, often via hydrogen bonding. Water is the most common protic solvent. Conversely, polar aprotic solvents cannot donate protons but still have the ability to dissolve many salts. Methods for purification of common solvents are available See also * Autoprotolysis In chemistry, autoprotolysis is a molecular autoionization, a chemical reaction in which a proton is transferred between two identical molecules, one of which acts as a Brønsted acid, releasing a proton that is accepted by the other molecule, wh ... References {{Chemical solutions Solvents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frustrated Lewis Pair
A frustrated Lewis pair (FLP) is a compound or mixture containing a Lewis acid and a Lewis base that, because of steric hindrance, cannot combine to form a classical adduct. Many kinds of FLPs have been devised, and many simple substrates exhibit activation. The discovery that some FLPs split H2 triggered a rapid growth of research into FLPs. Because of their "unquenched" reactivity, such systems are reactive toward substrates that can undergo heterolysis. For example, many FLPs split hydrogen molecules. Thus, a mixture of tricyclohexylphosphine (PCy3) and tris(pentafluorophenyl)borane reacts with hydrogen to give the respective phosphonium and borate ions: : This reactivity has been exploited to produce FLPs which catalyse hydrogenation reactions. Small molecule activation Frustrated Lewis pairs have been shown to activate many small molecules, either by inducing heterolysis or by coordination. Hydrogen The discovery that some FLPs are able to split, and therefore a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same cataly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gutmann–Beckett Method
In chemistry, the Gutmann–Beckett method is an experimental procedure used by chemists to assess the Lewis acidity of molecular species. Triethylphosphine oxide (, TEPO) is used as a probe molecule and systems are evaluated by 31P-NMR spectroscopy. In 1975, used 31P-NMR spectroscopy to parameterize Lewis acidity of solvents by acceptor numbers (AN).U. Mayer, V. Gutmann, and W. Gerger, "The acceptor number – a quantitative empirical parameter for the electrophilic properties of solvents", ''Monatshefte fur Chemie,'' 1975, 106, 1235–1257. doi: 10.1007/BF00913599 In 1996, Michael A. Beckett recognised its more generally utility and adapted the procedure so that it could be easily applied to molecular species, when dissolved in weakly Lewis acidic solvents.M.A. Beckett, G.C. Strickland, J.R. Holland, and K.S. Varma, "A convenient NMR method for the measurement of Lewis acidity at boron centres: correlation of reaction rates of Lewis acid initiated epoxide polymerizations wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition State
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked with the double dagger (‡) symbol. As an example, the transition state shown below occurs during the SN2 reaction of bromoethane with a hydroxide anion: The activated complex of a reaction can refer to either the transition state or to other states along the reaction coordinate between reactants and products, especially those close to the transition state. Peter Atkins and Julio de Paula, ''Physical Chemistry'' (8th ed., W.H. Freeman 2006), p.809 According to the transition state theory, once the reactants have passed through the transition state configuration, they always continue to form products. History of concept The concept of a transition state has been important in many theories of the rates at which chemical re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
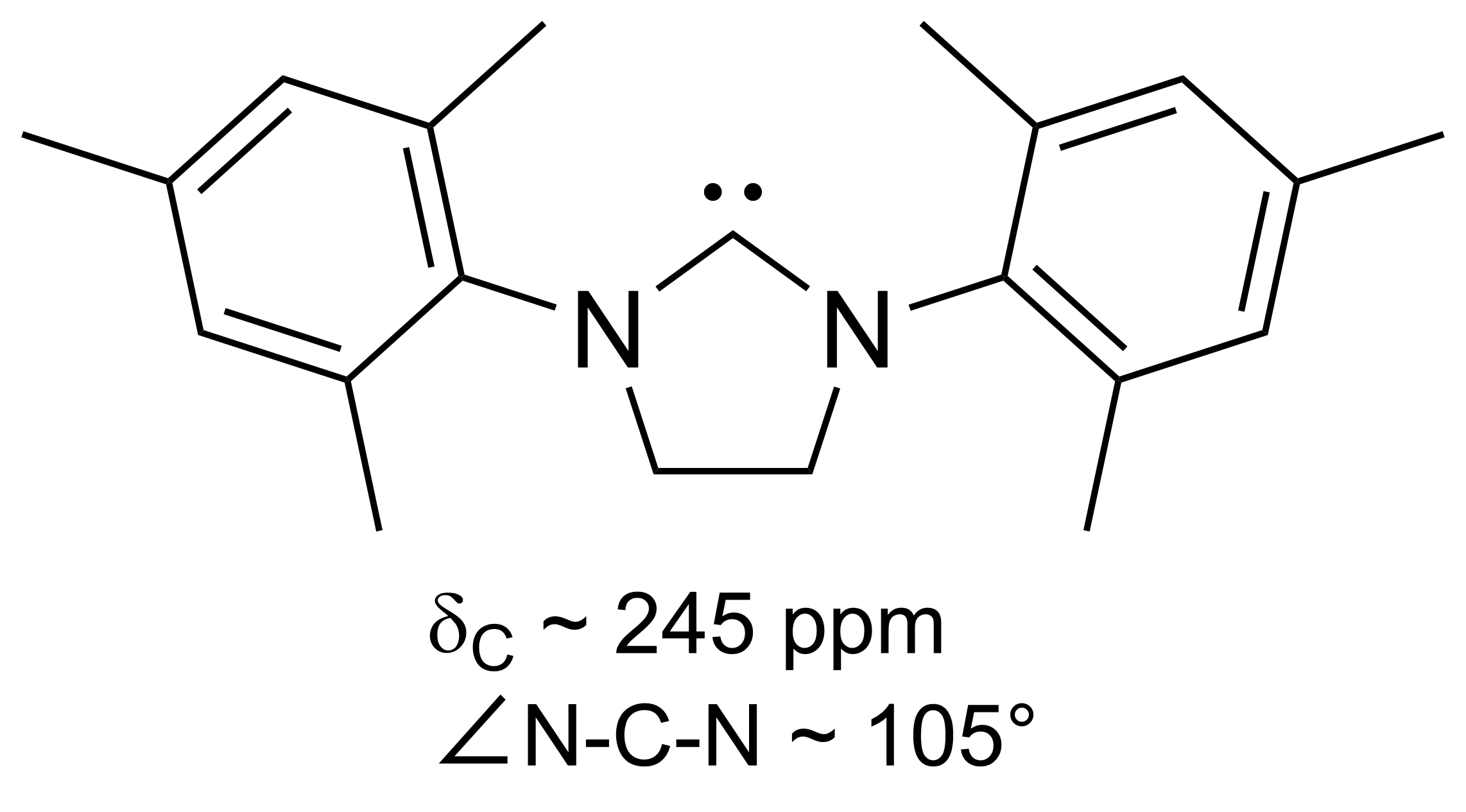
N-heterocyclic Carbene
A persistent carbene (also known as stable carbene) is an organic molecule whose natural resonance structure has a carbon atom with octet rule, incomplete octet (a carbene), but does not exhibit the tremendous instability typically associated with such moieties. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), in which nitrogen atoms flank the formal carbene. Modern theoretical analysis suggests that the term "persistent carbene" is in fact a misnomer. Persistent carbenes do not in fact have a carbene electronic structure in their ground state, but instead an ylide stabilized by Aromaticity, aromatic resonance or steric shielding. Excitation to a carbene structure then accounts for the carbene-like dimerization that some persistent carbenes undergo over the course of days. Persistent carbenes in general, and Arduengo carbenes in particular, are popular ligands in organometallic chemistry. Histor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bh2nhc Nocv
BH, Bh or bh may refer to: Medicine * Bernard-Horner syndrome, a combination of symptoms that arises when a group of nerves known as the sympathetic trunk is damaged * Borderline hypertensive, an American medical classification for cases where a person's blood pressure is elevated above normal, but not to the level considered hypertension * Bronchial hyperresponsiveness, a state characterised by easily triggered bronchospasm * Bundle of His, collection of heart muscle cells specialized for electrical conduction Science and technology * BH register, in computer architectures * Bohrium, symbol Bh, a chemical element * Boron monohydride, chemical formula BH, a chemical compound * Black hole Places * BH postcode area, a region in southern England served by Bournemouth postal sorting office * Bahrain (ISO 3166-1 country code BH) ** .bh, the Internet country code top-level domain for Bahrain * Belize's WMO and obsolete NATO country code digram * Belo Horizonte, the capital of Min ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
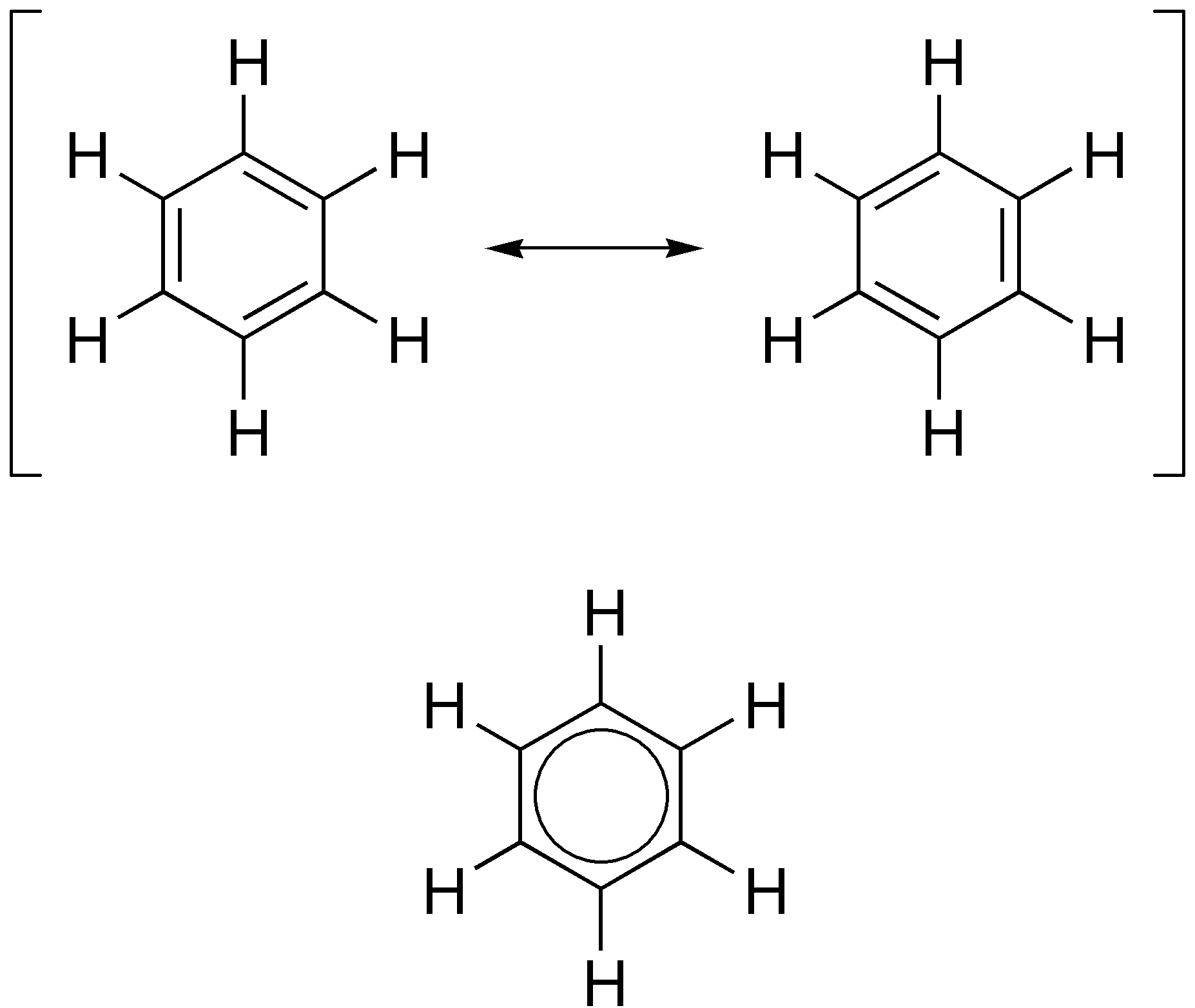
Aromaticity
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double- bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). Each bond may be seen as a hybrid of a single bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
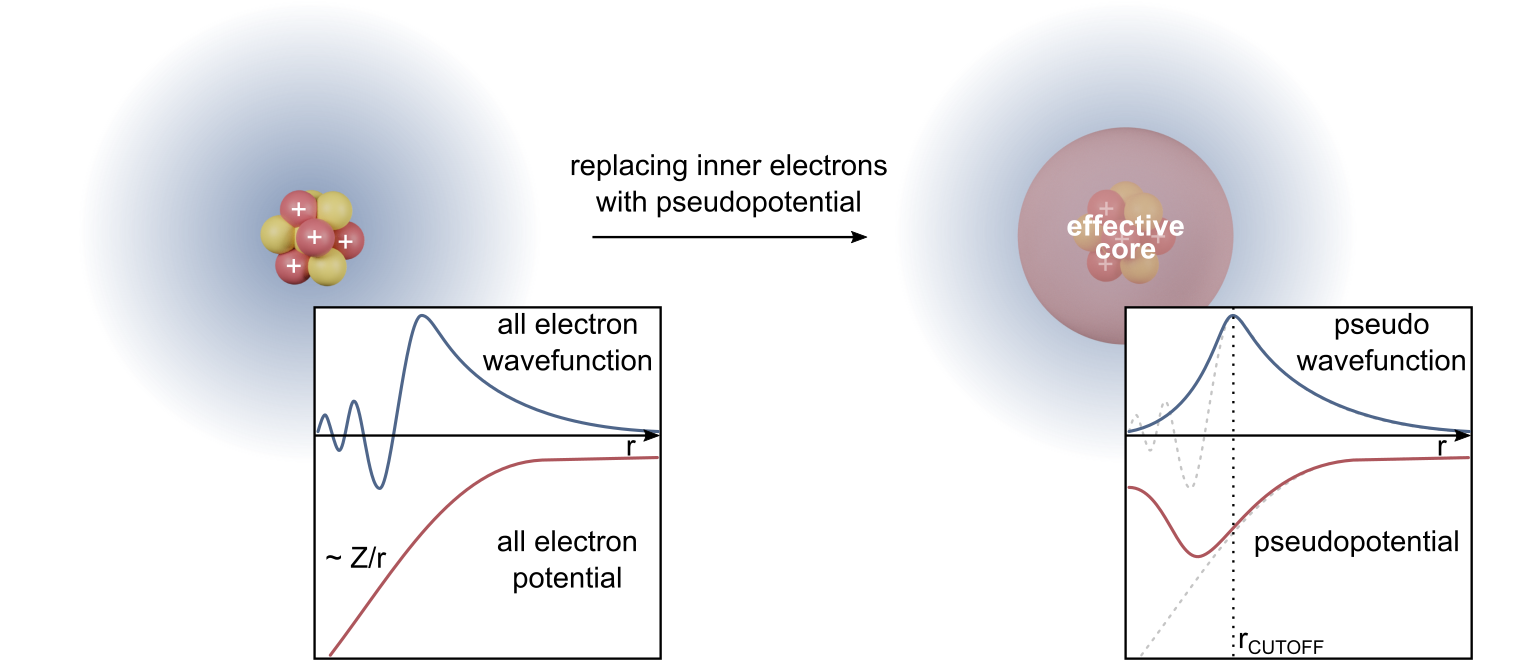
Density Functional Theory
Density functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body systems, in particular atoms, molecules, and the condensed phases. Using this theory, the properties of a many-electron system can be determined by using functionals - that is, functions that accept a function as input and output a single real number. In the case of DFT, these are functionals of the spatially dependent electron density. DFT is among the most popular and versatile methods available in condensed-matter physics, computational physics, and computational chemistry. DFT has been very popular for calculations in solid-state physics since the 1970s. However, DFT was not considered accurate enough for calculations in quantum chemistry until the 1990s, when the approximations used in the theory were greatly refined to better m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |