|
Binding Site
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may include other proteins (resulting in a protein–protein interaction), enzyme substrates, second messengers, hormones, or allosteric modulators. The binding event is often, but not always, accompanied by a conformational change that alters the protein's function. Binding to protein binding sites is most often reversible (transient and non-covalent), but can also be covalent reversible or irreversible. Function Binding of a ligand to a binding site on protein often triggers a change in conformation in the protein and results in altered cellular function. Hence binding site on protein are critical parts of signal transduction pathways. Types of ligands include neurotransmitters, toxins, neuropeptides, and steroid hormones. Binding site ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Glucose Binding Hexokinase
Glucose is a sugar with the molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight. It is used by plants to make cellulose, the most abundant carbohydrate in the world, for use in cell walls, and by all living organisms to make adenosine triphosphate (ATP), which is used by the cell as energy. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as amylose and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form is -glucose, while its stereoisomer -glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Steroid Hormone
A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids (typically made in the adrenal cortex, hence ''cortico-'') and sex steroids (typically made in the gonads or placenta). Within those two classes are five types according to the receptors to which they bind: glucocorticoids and mineralocorticoids (both corticosteroids) and androgens, estrogens, and progestogens (sex steroids). Vitamin D derivatives are a sixth closely related hormone system with homologous receptors. They have some of the characteristics of true steroids as receptor ligands. Steroid hormones help control metabolism, inflammation, immune functions, salt and water balance, development of sexual characteristics, and the ability to withstand injury and illness. The term steroid describes both hormones produced by the body and artificially produced medications that duplicate the action for the naturally occurring steroids. Synthesis The natu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Druggability
Druggability is a term used in drug discovery to describe a biological target (such as a protein) that is known to or is predicted to bind with high affinity to a drug. Furthermore, by definition, the binding of the drug to a druggable target must alter the function of the target with a therapeutic benefit to the patient. The concept of druggability is most often restricted to small molecules (low molecular weight organic substances) but also has been extended to include biologic medical products such as therapeutic monoclonal antibodies. Drug discovery comprises a number of stages that lead from a biological hypothesis to an approved drug. Target identification is typically the starting point of the modern drug discovery process. Candidate targets may be selected based on a variety of experimental criteria. These criteria may include disease linkage (mutations in the protein are known to cause a disease), mechanistic rationale (for example, the protein is part of a regulatory ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Catabolism
Catabolism () is the set of metabolic pathways that breaks down molecules into smaller units that are either oxidized to release energy or used in other anabolic reactions. Catabolism breaks down large molecules (such as polysaccharides, lipids, nucleic acids, and proteins) into smaller units (such as monosaccharides, fatty acids, nucleotides, and amino acids, respectively). Catabolism is the breaking-down aspect of metabolism, whereas anabolism is the building-up aspect. Cells use the monomers released from breaking down polymers to either construct new polymer molecules or degrade the monomers further to simple waste products, releasing energy. Cellular wastes include lactic acid, acetic acid, carbon dioxide, ammonia, and urea. The formation of these wastes is usually an oxidation process involving a release of chemical free energy, some of which is lost as heat, but the rest of which is used to drive the synthesis of adenosine triphosphate (ATP). This molecule acts as a way ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phosphofructokinase
Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis. Function The enzyme-catalysed transfer of a phosphoryl group from ATP is an important reaction in a wide variety of biological processes. Phosphofructokinase catalyses the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate, a key regulatory step in the glycolytic pathway. It is allosterically inhibited by ATP and allosterically activated by AMP, thus indicating the cell's energetic needs when it undergoes the glycolytic pathway. PFK exists as a homotetramer in bacteria and mammals (where each monomer possesses 2 similar domains) and as an octomer in yeast (where there are 4 alpha- (PFK1) and 4 beta-chains (PFK2), the latter, like the mammalian monomers, possessing 2 similar domains). This protein may use the morpheein model of allosteric regulation. PFK is about 300 amino acids in length, and structural studies of the bacterial enzyme have show ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Allosteric Regulation
In the fields of biochemistry and pharmacology an allosteric regulator (or allosteric modulator) is a substance that binds to a site on an enzyme or receptor distinct from the active site, resulting in a conformational change that alters the protein's activity, either enhancing or inhibiting its function. In contrast, substances that bind directly to an enzyme's active site or the binding site of the endogenous ligand of a receptor are called orthosteric regulators or modulators. The site to which the effector binds is termed the ''allosteric site'' or ''regulatory site''. Allosteric sites allow effectors to bind to the protein, often resulting in a conformational change and/or a change in protein dynamics. Effectors that enhance the protein's activity are referred to as ''allosteric activators'', whereas those that decrease the protein's activity are called ''allosteric inhibitors''. Allosteric regulations are a natural example of control loops, such as feedback from do ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Enzyme Inhibition
An enzyme inhibitor is a molecule that binds to an enzyme and blocks its Enzyme activity, activity. Enzymes are proteins that speed up chemical reactions necessary for life, in which Substrate (biochemistry), substrate molecules are converted into Product (chemistry), products. An enzyme Enzyme catalysis, facilitates a specific chemical reaction by binding the substrate to its active site, a specialized area on the enzyme that accelerates the Rate-determining step, most difficult step of the reaction. An enzyme inhibitor stops ("inhibits") this process, either by binding to the enzyme's active site (thus preventing the substrate itself from binding) or by binding to another site on the enzyme such that the enzyme's catalysis of the reaction is blocked. Enzyme inhibitors may bind Reversible reaction, reversibly or irreversibly. Irreversible inhibitors form a Covalent bond, chemical bond with the enzyme such that the enzyme is inhibited until the chemical bond is broken. By cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prosthetic group, component of hemoglobin, which is necessary to bind oxygen in the bloodstream. It is composed of four pyrrole rings with 2 Vinyl group, vinyl and 2 propionic acid side chains. Heme is biosynthesized in both the bone marrow and the liver. Heme plays a critical role in multiple different redox reactions in mammals, due to its ability to carry the oxygen molecule. Reactions include oxidative metabolism (cytochrome c oxidase, succinate dehydrogenase), xenobiotic detoxification via cytochrome P450 pathways (including Drug metabolism, metabolism of some drugs), gas sensing (Guanylate cyclase, guanyl cyclases, nitric oxide synthase), and microRNA processing (DGCR8). Heme is a coordination complex "consisting of an iron ion coordinated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
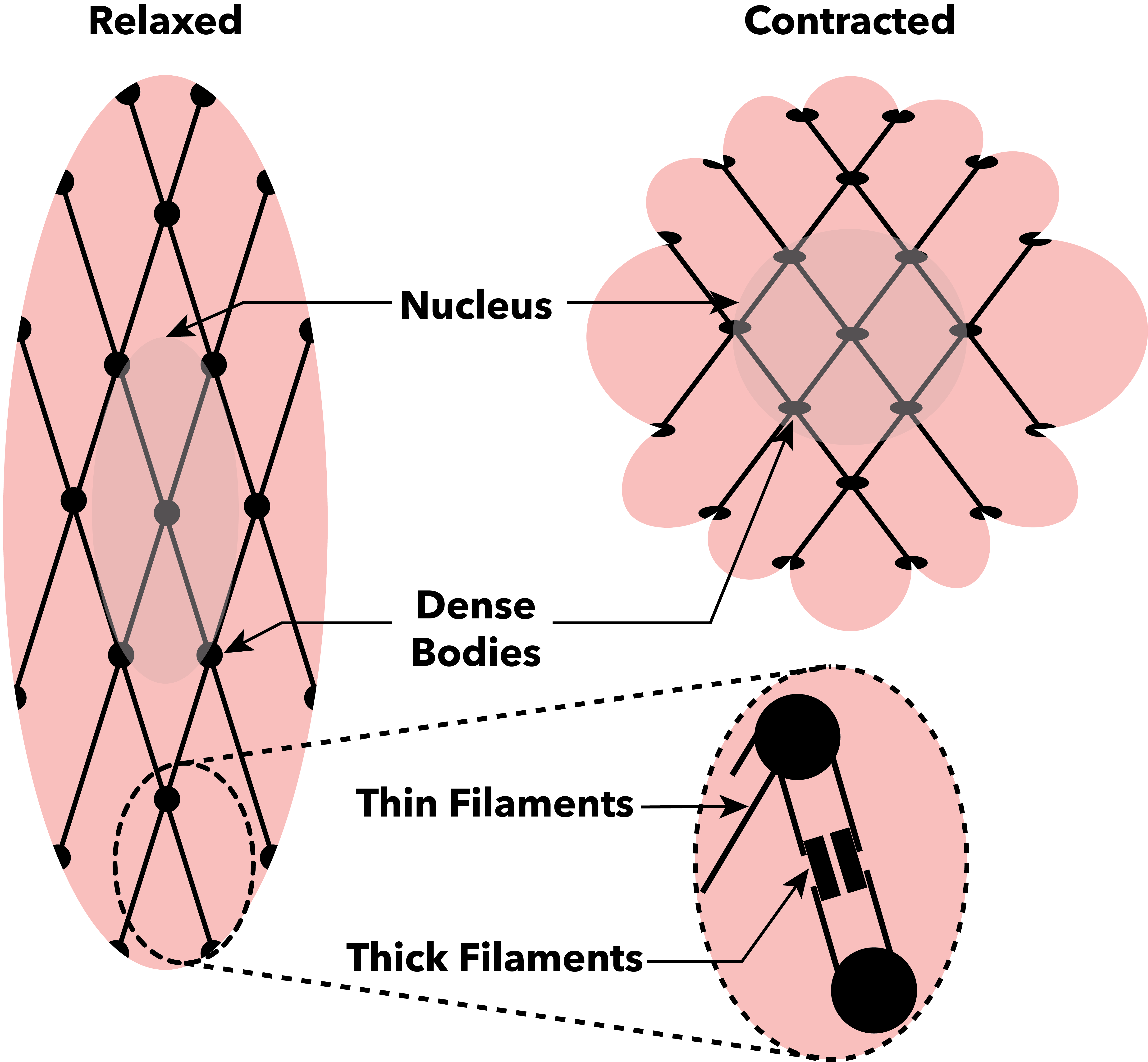
Muscle Contraction
Muscle contraction is the activation of Tension (physics), tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such as when holding something heavy in the same position. The termination of muscle contraction is followed by muscle relaxation, which is a return of the muscle fibers to their low tension-generating state. For the contractions to happen, the muscle cells must rely on the change in action of two types of Myofilament, filaments: thin and thick filaments. The major constituent of thin filaments is a chain formed by helical coiling of two strands of actin, and thick filaments dominantly consist of chains of the Motor protein, motor-protein myosin. Together, these two filaments form myofibrils - the basic functional organelles in the skeletal muscle system. In vertebrates, Muscle cell#Muscle contraction in striated muscle, skele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Sliding Filament Theory
The sliding filament theory explains the mechanism of muscle contraction based on muscle proteins that slide past each other to generate movement. According to the sliding filament theory, the myosin ( thick filaments) of muscle fibers slide past the actin ( thin filaments) during muscle contraction, while the two groups of filaments remain at relatively constant length. The theory was independently introduced in 1954 by two research teams, one consisting of Andrew Huxley and Rolf Niedergerke from the University of Cambridge, and the other consisting of Hugh Huxley and Jean Hanson from the Massachusetts Institute of Technology. It was originally conceived by Hugh Huxley in 1953. Andrew Huxley and Niedergerke introduced it as a "very attractive" hypothesis. Before the 1950s there were several competing theories on muscle contraction, including electrical attraction, protein folding, and protein modification. The novel theory directly introduced a new concept called cross-bridg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Uncompetitive Inhibitor
Uncompetitive inhibition (which Laidler and Bunting preferred to call anti-competitive inhibition, but this term has not been widely adopted) is a type of enzyme inhibition in which the apparent values of the Michaelis–Menten parameters V and K_\mathrm are decreased in the same proportion. It can be recognized by two observations: first, it cannot be reversed by increasing the substrate concentration a, and second, linear plots show effects on V and K_\mathrm, seen, for example, in the Lineweaver–Burk plot as parallel rather than intersecting lines. It is sometimes explained by supposing that the inhibitor can bind to the enzyme-substrate complex but not to the free enzyme. This type of mechanism is rather rare, and in practice uncompetitive inhibition is mainly encountered as a limiting case of inhibition in two-substrate reactions in which one substrate concentration is varied and the other is held constant at a saturating level. Mathematical definition In uncompetitive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Competitive Inhibition
Competitive inhibition is interruption of a chemistry, chemical pathway owing to one chemical substance inhibiting the effect of another by competing with it for molecular binding, binding or chemical bond, bonding. Any metabolism, metabolic or chemical messenger (other), chemical messenger system can potentially be affected by this principle, but several classes of competitive inhibition are especially important in biochemistry and medicine, including the competitive form of enzyme inhibitor, enzyme inhibition, the competitive form of receptor antagonist, receptor antagonism, the competitive form of antimetabolite activity, and the competitive form of poisoning (which can include any of the aforementioned types). Enzyme inhibition type In competitive inhibition of enzyme catalysis, binding of an inhibitor prevents binding of the target molecule of the enzyme, also known as the substrate. This is accomplished by blocking the binding site of the substrate – the active si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |