|
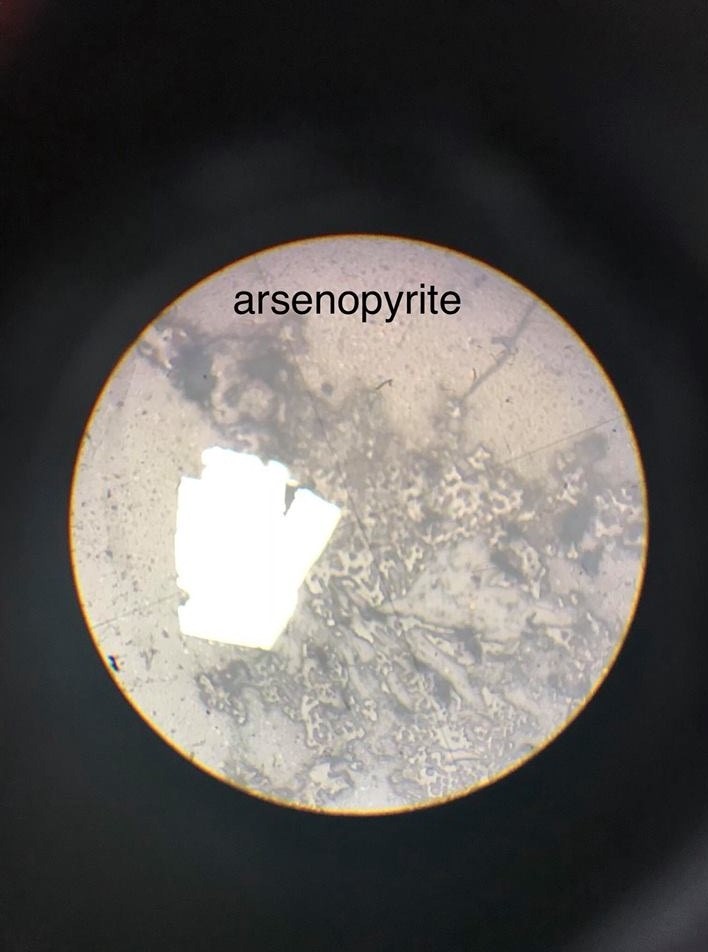
Arsenopyrite
Arsenopyrite ( IMA symbol: Apy) is an iron arsenic sulfide (FeAsS). It is a hard ( Mohs 5.5-6) metallic, opaque, steel grey to silver white mineral with a relatively high specific gravity of 6.1. When dissolved in nitric acid, it releases elemental sulfur. When arsenopyrite is heated, it produces sulfur and arsenic vapor. With 46% arsenic content, arsenopyrite, along with orpiment, is a principal ore of arsenic. When deposits of arsenopyrite become exposed to the atmosphere, the mineral slowly converts into iron arsenates. Arsenopyrite is generally an acid-consuming sulfide mineral, unlike iron pyrite which can lead to acid mine drainage. The crystal habit, hardness, density, and garlic odour when struck are diagnostic. Arsenopyrite in older literature may be referred to as ''mispickel'', a name of German origin. Arsenopyrite also can be associated with significant amounts of gold. Consequently, it serves as an indicator of gold bearing reefs. Many arsenopyrite gold ores ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenopyrite By Petrographic Microscope NL
Arsenopyrite (International Mineralogical Association, IMA List of mineral symbols, symbol: Apy) is an iron arsenic sulfide (FeAsS). It is a hard (Mohs scale of mineral hardness, Mohs 5.5-6) metallic, opaque, steel grey to silver white mineral with a relatively high specific gravity of 6.1. When dissolved in nitric acid, it releases elemental sulfur. When arsenopyrite is heated, it produces sulfur and arsenic vapor. With 46% arsenic content, arsenopyrite, along with orpiment, is a principal ore of arsenic. When deposits of arsenopyrite become exposed to the atmosphere, the mineral slowly converts into iron arsenates. Arsenopyrite is generally an acid-consuming sulfide mineral, unlike pyrite, iron pyrite which can lead to acid mine drainage. The crystal habit, hardness, density, and garlic odour when struck are diagnostic. Arsenopyrite in older literature may be referred to as ''mispickel'', a name of German origin. Arsenopyrite also can be associated with significant amount ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrite
The mineral pyrite (), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Iron, FeSulfur, S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral. Pyrite's metallic Luster (mineralogy), luster and pale brass-yellow hue give it a superficial resemblance to gold, hence the well-known nickname of ''fool's gold''. The color has also led to the nicknames ''brass'', ''brazzle'', and ''Brazil'', primarily used to refer to pyrite found in coal. The name ''pyrite'' is derived from the Greek language, Greek (), 'stone or mineral which strikes fire', in turn from (), 'fire'. In ancient Roman times, this name was applied to several types of stone that would create sparks when struck against steel; Pliny the Elder described one of them as being brassy, almost certainly a reference to what we now call pyrite. By Georgius Agricola's time, , the term had become a generic term for all of the pyrite group, sulfide minerals. Pyrite is usua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but only the gray form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead (for example, in car batteries and ammunition). Arsenic is a common n-type dopant in semiconductor electronic devices. It is also a component of the III-V compound semiconductor gallium arsenide. Arsenic and its compounds, especially the trioxide, are used in the production of pesticides, treated wood products, herbicides, and insecticides. These applications are declining with the increasing recognition of the toxicity of arsenic and its compounds. A few species of bacteria are able to use arsenic compounds as respiratory metabolites. Trace quantities of arsenic are an essential dietary element in rats, ham ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfide Mineral
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide (S22−) as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenides, the tellurides, the arsenides, the antimonides, the bismuthinides, the sulfarsenides and the sulfosalts.http://www.minerals.net/mineral/sort-met.hod/group/sulfgrp.htm Minerals.net Dana Classification, SulfidesKlein, Cornelis and Cornelius S. Hurlbut, Jr., 1986, ''Manual of Mineralogy'', Wiley, 20th ed., pp 269-293 Sulfide minerals are inorganic compounds. Minerals Common or important examples include: * Acanthite *Chalcocite *Bornite * Galena * Sphalerite *Chalcopyrite *Pyrrhotite *Millerite *Pentlandite *Covellite *Cinnabar *Realgar *Orpiment *Stibnite *Pyrite *Marcasite *Molybdenite Sulfarsenides: *Cobaltite *Arsenopyrite *Gersdorffite Sulfosalts: *Pyrargyrite *Proustite *Tetrahedrite *Tennantite *Enargite *Bournonite * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pegmatite
A pegmatite is an igneous rock showing a very coarse texture, with large interlocking crystals usually greater in size than and sometimes greater than . Most pegmatites are composed of quartz, feldspar, and mica, having a similar silicic composition to granite. However, rarer intermediate composition and mafic pegmatites are known. Many of the world's largest crystals are found within pegmatites. These include crystals of microcline, quartz, mica, spodumene, beryl, and tourmaline. Some individual crystals are over long. Most pegmatites are thought to form from the last fluid fraction of a large crystallizing magma body. This residual fluid is highly enriched in volatiles and trace elements, and its very low viscosity allows molecules to migrate rapidly to join an existing crystal rather than coming together to form new crystals. This allows a few very large crystals to form. While most pegmatites have a simple composition of minerals common in ordinary igneous rock, a few ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can use d orbitals as valence orbitals to form chemical bonds. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. Since they are metals, they are lustrous and have good electrical and thermal conductivity. Most (with the exception of group 11 and group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such as coordination complexes and oxides. Most are strongly param ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term ''covalent bond'' dates from 1939. The prefix ''co-'' means ''jointly, associated in action, partnered to a lesser degree, '' etc.; thus a "co-valent bond", in essence, means that the atoms share " valence", such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marcasite
The mineral marcasite, sometimes called “white iron pyrite”, is iron sulfide (FeS2) with orthorhombic crystal structure. It is physically and crystallographically distinct from pyrite, which is iron sulfide with cubic crystal structure. Both structures do have in common that they contain the disulfide S22− ion, having a short bonding distance between the sulfur atoms. The structures differ in how these di-anions are arranged around the Fe2+ cations. Marcasite is lighter and more brittle than pyrite. Specimens of marcasite often crumble and break up due to the unstable crystal structure. On fresh surfaces, it is pale yellow to almost white and has a bright metallic luster. It tarnishes to a yellowish or brownish color and gives a black streak. It is a brittle material that cannot be scratched with a knife. The thin, flat, tabular crystals, when joined in groups, are called “cockscombs”. In marcasite jewellery, pyrite used as a gemstone is called “marcasite” – t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal. Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , row oC, column Primitive, where the cell parameters are given as a1 = a2, α = β = 90° it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Twinning
Crystal twinning occurs when two or more adjacent crystals of the same mineral are oriented so that they share some of the same crystal lattice points in a symmetrical manner. The result is an intergrowth of two separate crystals that are tightly bonded to each other. The surface along which the lattice points are shared in twinned crystals is called a composition surface or twin plane. Crystallographers classify twinned crystals by a number of twin laws. These twin laws are specific to the crystal structure. The type of twinning can be a diagnostic tool in mineral identification. Deformation twinning, in which twinning develops in a crystal in response to a shear stress, is an important mechanism for permanent shape changes in a crystal.Courtney, Thomas H. (2000) ''Mechanical Behavior of Materials'', 2nd ed. McGraw Hill. Definition Twinning is a form of symmetrical intergrowth between two or more adjacent crystals of the same mineral. It differs from the ordinary random i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Striation (geology)
In geology, a striation is a groove, created by a geological process, on the surface of a rock or a mineral. In structural geology, striations are linear furrows, or linear marks, generated from fault movement. The striation's direction reveals the movement direction in the fault plane. Similar striations, called glacial striations, can occur in areas subjected to glaciation. Striations can also be caused by underwater landslides. Striations can also be a growth pattern or mineral habit that looks like a set of hairline grooves, seen on crystal faces of certain minerals. Examples of minerals that can show growth striations include pyrite, feldspar, quartz, tourmaline, chalcocite and sphalerite. Glacial Striations The surface of rocks can have an altered appearance as a result of the movement of ice. They can show a polished looking surface scarred with glacial striations. Often these striations carved into the bedrock extend for long distances. The scars are a result of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal System
In crystallography, a crystal system is a set of point groups (a group of geometric symmetries with at least one fixed point). A lattice system is a set of Bravais lattices. Space groups are classified into crystal systems according to their point groups, and into lattice systems according to their Bravais lattices. Crystal systems that have space groups assigned to a common lattice system are combined into a crystal family. The seven crystal systems are triclinic, monoclinic, orthorhombic, tetragonal, trigonal, hexagonal, and cubic. Informally, two crystals are in the same crystal system if they have similar symmetries (albeit there are many exceptions). Classifications Crystals can be classified in three ways: lattice systems, crystal systems and crystal families. The various classifications are often confused: in particular the trigonal crystal system is often confused with the rhombohedral lattice system, and the term "crystal system" is sometimes used to mean "latti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |