|
Aminoacetonitrile
Aminoacetonitrile is the organic compound with the formula NCCH2NH2. The compound is a colorless liquid. It is unstable at room temperature, owing to the incompatibility of the amine nucleophile and the nitrile electrophile. For this reason it is usually encountered as the chloride and bisulfate salts of the ammonium derivative, i.e., CCH2NH3sup>+Cl− and CCH2NH3sup>+HSO4−. Production and applications Industrially aminoacetonitrile is produced from glycolonitrile by reaction with ammonia: :HOCH2CN + NH3 → H2NCH2CN + H2O The aminoacetonitrile can be hydrolysed to give glycine: Being bifunctional, it is useful in the synthesis of diverse nitrogen-containing heterocycles. Aminoacetonitrile derivatives are useful antihelmintics. They act as nematode specific ACh agonists causing a spastic paralysis and rapid expulsion from the host. Occurrence in the interstellar medium Using radio astronomy, aminoacetonitrile was discovered in the Large Molecule Heimat, a giant gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolonitrile
Glycolonitrile, also called hydroxyacetonitrile or formaldehyde cyanohydrin, is the organic compound with the formula HOCH2CN. It is the simplest cyanohydrin and it is derived from formaldehyde. It is a colourless liquid that dissolves in water and ether. Because glycolonitrile decomposes readily into formaldehyde and hydrogen cyanide, it is listed as an extremely hazardous substance. In January 2019, astronomers reported the detection of glycolonitrile, another possible building block of life among other such molecules, in outer space. Synthesis and reactions Glycolonitrile is produced by reacting formaldehyde with hydrogen cyanide under acidic conditions. This reaction is catalysed by base..Peter Pollak, Gérard Romeder, Ferdinand Hagedorn, Heinz-Peter Gelbke "Nitriles" ''Ullmann's Encyclopedia of Industrial Chemistry'' 2002, Wiley-VCH, Weinheim. Glycolonitrile polymerizes under alkaline conditions above pH 7.0. As the product of polymerization is an amine with a basic char ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Large Molecule Heimat
The Large Molecule Heimat is a dense gas cloud located in the molecular cloud Sagittarius B2. Many species of molecule, including aminoacetonitrile (a molecule related to glycine), ethyl formate, and butyronitrile Butyronitrile or butanenitrile or propyl cyanide, is a nitrile with the formula C3H7CN. This colorless liquid is miscible with most polar organic solvents. Uses Butyronitrile is mainly used as a precursor to the poultry drug amprolium.Peter Pol ..., have been detected in the Large Molecule Heimat. References Milky Way Astrochemistry Sagittarius (constellation) {{nebula-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to its compact form. For the same reason, it is the most abundant amino acid in collagen triple-helices. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a ''Clostridium tetani'' infection) can cause spastic paralysis due to uninhibited muscle contraction. It is the only achiral proteinogenic amino acid. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom. History and etymology Glycine was discovered in 1820 by the French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Institute Of Standards And Technology
The National Institute of Standards and Technology (NIST) is an agency of the United States Department of Commerce whose mission is to promote American innovation and industrial competitiveness. NIST's activities are organized into physical science laboratory programs that include nanoscale science and technology, engineering, information technology, neutron research, material measurement, and physical measurement. From 1901 to 1988, the agency was named the National Bureau of Standards. History Background The Articles of Confederation, ratified by the colonies in 1781, provided: The United States in Congress assembled shall also have the sole and exclusive right and power of regulating the alloy and value of coin struck by their own authority, or by that of the respective states—fixing the standards of weights and measures throughout the United States. Article 1, section 8, of the Constitution of the United States, ratified in 1789, granted these powers to the new ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
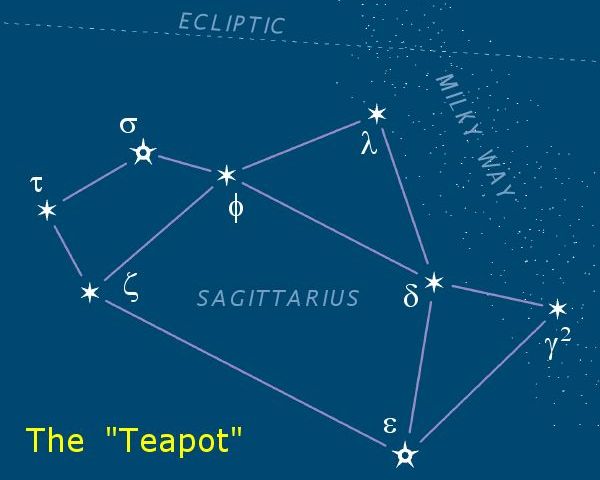
Sagittarius (constellation)
Sagittarius is one of the constellations of the zodiac and is located in the Southern celestial hemisphere. It is one of the 48 constellations listed by the 2nd-century astronomer Ptolemy and remains one of the 88 modern constellations. Its old astronomical symbol is (♐︎). Its name is Latin for "archer". Sagittarius is commonly represented as a centaur pulling back a bow. It lies between Scorpius and Ophiuchus to the west and Capricornus and Microscopium to the east. The center of the Milky Way lies in the westernmost part of Sagittarius (see Sagittarius A). Visualizations As seen from the northern hemisphere, the constellation's brighter stars form an easily recognizable asterism known as "the Teapot". The stars δ Sgr (Kaus Media), ε Sgr (Kaus Australis), ζ Sgr (Ascella), and φ Sgr form the body of the pot; λ Sgr (Kaus Borealis) is the point of the lid; γ2 Sgr (Alnasl) is the tip of the spout; and σ Sgr (Nunki) and τ Sgr the handle. These ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Max Planck Institute For Radio Astronomy
The Max Planck Institute for Radio Astronomy (MPIfRA) (German: ''Max-Planck-Institut für Radioastronomie'') is located in Bonn, Germany. It is one of 80 institutes in the Max Planck Society (German: Max-Planck-Gesellschaft). History By combining the already existing radio astronomy faculty of the University of Bonn led by Otto Hachenberg with the new Max Planck institute the Max Planck Institute for Radio Astronomy was formed. In 1972 the 100-m radio telescope in Effelsberg was opened. The institute building was enlarged in 1983 and 2002. The southern wing of the whole complex is occupied by the Argelander Institute of Astronomy of the University of Bonn. Structure The Institute has three main research groups, each with its own Director Departments * Fundamental Physics ( Michael Kramer) * VLBI and Radio Astronomy (Anton Zensus) * Millimetre Astronomy ( Karl Menten) Independent Research Groups * Lise Meitner Group on Fast Radio Bursts as Astrophysical Tools ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by organic groups (indicated by R). Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted acids (proton donors): :H+ + NH3 -> H4 The ammonium ion is mildly acidic, reacting with Brønsted bases to return to the uncharged ammonia molecule: : H4 + B- -> HB + NH3 Thus, treatment of concentrated solutions of ammonium salts with strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions: :H2O + NH3 OH- + H4 The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society (professional association) in the United Kingdom with the goal of "advancing the chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 34,000 in the UK and a further 8,000 abroad. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, China and in Ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Liv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DBNPA
DBNPA or 2,2-dibromo-3-nitrilopropionamide is a quick-kill biocide that easily hydrolyzes under both acidic and alkaline conditions. It is preferred for its instability in water as it quickly kills and then quickly degrades to form a number of products, depending on the conditions, including ammonia, bromide ions, dibromoacetonitrile, and dibromoacetic acid. DBNPA acts similar to the typical halogen biocides. DBNPA is used in a wide variety of applications. Some examples are in papermaking as a preservative in paper coating and slurries. It is also used as slime control on papermachines, and as a biocide in hydraulic fracturing Fracking (also known as hydraulic fracturing, hydrofracturing, or hydrofracking) is a well stimulation technique involving the fracturing of bedrock formations by a pressurized liquid. The process involves the high-pressure injection of "fra ... wells and in cooling water. References {{Reflist Antimicrobials Organobromides Acetamides Nitri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |