|
Zirconium Oxide
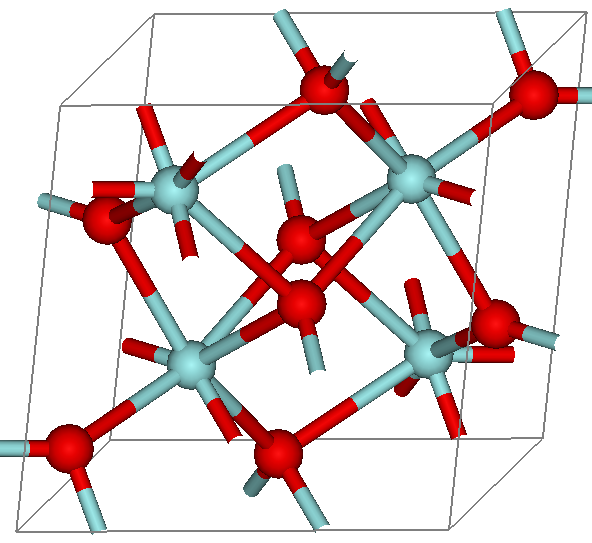
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabilized cubic structured zirconia, cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant. Production, chemical properties, occurrence Zirconia is produced by calcining zirconium compounds, exploiting its high thermostability.Ralph Nielsen "Zirconium and Zirconium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. Structure Three phases are known: monoclinic below 1170 °C, tetragonal between 1170 °C and 2370 °C, and cubic above 2370 °C. The trend is for higher symmetry at higher temperatures, as is usually the case. A small percentage of the oxides of calcium or yttrium stabilize in the cubic phase. The very rare mineral tazheran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baddeleyite
Baddeleyite is a rare zirconium oxide mineral (ZrO2 or zirconia), occurring in a variety of monoclinic prismatic crystal forms. It is transparent to translucent, has high indices of refraction, and ranges from colorless to yellow, green, and dark brown. See etymology below. Baddeleyite is a refractory mineral, with a melting point of 2700 °C. Hafnium is a substituting ''impurity'' and may be present in quantities ranging from 0.1 to several percent. It can be found in igneous rocks containing potassium feldspar and plagioclase. Baddeleyite is commonly not found with zircon (ZrSiO4), because it forms in silica-undersaturated rocks, such as mafic rocks. This is because, when silica is free in the system (silica-saturated/oversaturated), zircon is the dominating phase, not baddeleyite. It belongs to the monoclinic-prismatic class, of the P21/c crystal system. It has been used for geochronology. Geologic occurrence Baddeleyite was first found in Sri Lanka in 1892. It ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerium(III) Oxide
Cerium(III) oxide, also known as cerium oxide, cerium trioxide, cerium sesquioxide, cerous oxide or dicerium trioxide, is an oxide of the rare-earth metal cerium. It has chemical formula and is gold-yellow in color. Applications Engine and exhaust catalysts Cerium oxide is used as a catalytic converter for the minimisation of CO emissions in the exhaust gases from motor vehicles. When there is a shortage of oxygen, cerium(IV) oxide is reduced by carbon monoxide to cerium(III) oxide: : When there is an oxygen surplus, the process is reversed and cerium(III) oxide is oxidized to cerium(IV) oxide: : Major automotive applications for cerium(III) oxide are, as a catalytic converter for the oxidation of CO and emissions in the exhaust gases from motor vehicles, and secondly, cerium oxide finds use as a fuel additive to diesel fuels, which results in increased fuel efficiency and decreased hydrocarbon derived particulate matter emissions, however the health effects of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "'' lime''" connotes calcium-containing inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, ''quicklime'' specifically applies to the single chemical compound calcium oxide. Calcium oxide that survives processing without reacting in building products such as cement is called free lime. Quicklime is relatively inexpensive. Both it and a chemical derivative (calcium hydroxide, of which quicklime is the base anhydride) are important commodity chemicals. Preparation Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by heating the material to above ,Merck ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium(III) Oxide
Yttrium oxide, also known as yttria, is Y2 O3. It is an air-stable, white solid substance. The thermal conductivity of yttrium oxide is 27 W/(m·K). Uses Phosphors Yttria is widely used to make Eu:YVO4 and Eu:Y2O3 phosphors that give the red color in color TV picture tubes. Yttria lasers Y2O3 is a prospective solid-state laser material. In particular, lasers with ytterbium as dopant allow the efficient operation both in continuous operation and in pulsed regimes. At high concentration of excitations (of order of 1%) and poor cooling, the quenching of emission at laser frequency and avalanche broadband emission takes place. (Yttria-based lasers are not to be confused with YAG lasers using yttrium aluminum garnet, a widely used crystal host for rare earth laser dopants). Gas Lighting The original use of the mineral yttria and the purpose of its extraction from mineral sources was as part of the process of making gas mantles and other products for turning the flames of artifici ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from '' magnesia negra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Doping (semiconductor) In semiconductor production, doping is the intentional introduction of impurities into an intrinsic semiconductor for the purpose of modulating its electrical, optical and structural properties. The doped material is referred to as an extrinsic semiconductor. Small numbers of dopant atoms can change the ability of a semiconductor to conduct electricity. When on the order of one dopant atom is added per 100 million atoms, the doping is said to be ''low'' or ''light''. When many more dopant atoms are added, on the order of one per ten thousand atoms, the doping is referred to as ''high'' or ''heavy''. This is often shown as ''n+'' for n-type doping or ''p+'' for p-type doping. (''See the article on semiconductors for a more detailed description of the doping mechani |