|
Waelz Process
The Waelz process is a method of recovering zinc and other relatively low boiling point metals from metallurgical waste (typically electric arc furnace flue dust) and other recycled materials using a rotary kiln (''waelz kiln''). The zinc enriched product is referred to as ''waelz oxide'', and the reduced zinc by product as ''waelz slag''. History and description The concept of using a rotary kiln for the recovery of Zinc by volatization dates to at least 1888. A process was patented by Edward Dedolph in 1910. Subsequently, the Dedpolph patent was taken up and developed by Metallgesellschaft (Frankfurt) with Chemische Fabrik Griesheim-Elektron but without leading to a production scale ready process. In 1923 the Krupp Grusonwerk independently developed a process (1923), named the ''Waelz process'' (from the German ''Waelzen'', a reference to the motion of the materials in the kiln); the two German firms later collaborated and improved the process marketing under the name ''Waelz- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It is the second most abundant trace metal in humans after iron, an import ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Arc Furnace
An electric arc furnace (EAF) is a Industrial furnace, furnace that heats material by means of an electric arc. Industrial arc furnaces range in size from small units of approximately one-tonne capacity (used in foundry, foundries for producing cast iron products) up to about 400-tonne units used for secondary steelmaking. Arc furnaces used in research laboratories and by Dentistry, dentists may have a capacity of only a few dozen grams. Industrial electric arc furnace temperatures can reach , while laboratory units can exceed . In electric arc furnaces, the material inside the furnace (referred to as a charge) is directly exposed to an electric arc, and the current from the electrode terminals passes through the charge material. Arc furnaces differ from induction furnaces, which use eddy currents to heat the charge. History In the 19th century, a number of people had employed an electric arc to melt iron. Sir Humphry Davy conducted an experimental demonstration in 1810; we ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rotary Kiln
A rotary kiln is a pyroprocessing device used to raise materials to a high temperature (calcination) in a continuous process. Materials produced using rotary kilns include: * Cement * Lime * Refractories * Metakaolin * Titanium dioxide * Alumina * Vermiculite * Iron ore pellets They are also used for roasting a wide variety of sulfide ores prior to metal extraction. Principle of operation The kiln is a cylindrical vessel, inclined slightly from the horizontal, which is rotated slowly about its longitudinal axis. The process feedstock is fed into the upper end of the cylinder. As the kiln rotates, material gradually moves down toward the lower end, and may undergo a certain amount of stirring and mixing. Hot gases pass along the kiln, sometimes in the same direction as the process material (co-current), but usually in the opposite direction (counter-current). The hot gases may be generated in an external furnace, or may be generated by a flame inside the kiln. Su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallgesellschaft
Metallgesellschaft AG was formerly one of Germany's largest industrial conglomerates based in Frankfurt. It had over 20,000 employees and revenues in excess of 10 billion US dollars. It had over 250 subsidiaries specializing in mining, specialty chemicals (Chemetall), commodity trading, financial services, and engineering ( Lurgi). Henry Merton & Company, Ltd was previously a branch of the Metallgesellschaft. History Metallgesellschaft AG was incorporated in Frankfurt am Main in 1881 by Wilhelm Ralph Merton, his father Ralph Merton, and Leo Ellinger. Merton was responsible for business strategy, Ellinger for operations, and a cousin of Merton, Zachary Hochschild, for marketing and international activities. Their main competitors were the two other large metal trading companies of Germany: '' Aron Hirsch & Sohn'' in Halberstadt, and ''Beer, Sondheimer & Co'' in Frankfurt am Main. Although Metallgesellschaft was a joint stock company, it was operated like a family business with ke ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Krupp Grusonwerk
Friedrich Krupp AG Hoesch-Krupp (formerly Fried. Krupp AG and Friedrich Krupp GmbH), trading as Krupp, was the largest company in Europe at the beginning of the 20th century as well as Germany's premier weapons manufacturer during both world wars. It produced battleships, U-boats, tanks, howitzers, guns, utilities, and hundreds of other commodities. The company also produced steel used to build railroads in the United States and to cap the Chrysler Building. After the Nazis seized power in Germany, Krupp supported the regime and was one of many German businesses that profited from slave labor during World War II. Upon the war's end, the head of the company, Alfried Krupp, was tried and convicted as a war criminal for employing prisoners of war, foreign civilians and concentration camp inmates under inhumane conditions in support of the Nazi war effort. Despite being sentenced to imprisonment for twelve years, he served just three and was pardoned (but not acquitted) by J ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Oxide
Zinc oxide is an inorganic compound with the Chemical formula, formula . It is a white powder which is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, Zinc metabolism, food supplements, rubbers, plastics, ceramics, glass, cement, lubricants, paints, sunscreens, ointments, adhesives, sealants, pigments, foods, batteries, ferrites, fire retardants, semi conductors, and first-aid tapes. Although it occurs naturally as the mineral zincite, most zinc oxide is produced synthetically. History Early humans probably used zinc compounds in processed and unprocessed forms, as paint or medicinal ointment; however, their composition is uncertain. The use of ''pushpanjan'', probably zinc oxide, as a salve for eyes and open wounds is mentioned in the Indian medical text the Charaka Samhita, thought to date from 500 BC or before. Zinc oxide ointment is also mentioned by the Greek physician Dioscorides (1st century AD). Galen suggested treatin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Silicate (other)
Zinc silicate may refer to: * Hemimorphite Hemimorphite is the chemical compound Zinc, Zn4(Pyrosilicate, Si2O7)(Hydroxide, OH)2Water of crystallization, ·H2O, a component of mineral Calamine (mineral), calamine. It is a silicate mineral which, together with smithsonite (ZnCO3), has bee ..., a zinc sorosilicate * Willemite, a zinc neosilicate * Sauconite, a zinc phyllosilicate {{Short pages monitor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Ferrite
Zinc ferrites are a series of synthetic inorganic compounds of zinc and iron (ferrite) with the general formula of ZnxFe3−xO4. Zinc ferrite compounds can be prepared by aging solutions of Zn(NO3)2, Fe(NO3)3, and triethanolamine in the presence and in the absence of hydrazine, or reacting iron oxides and zinc oxide at high temperature. Spinel (Zn, Fe) Fe2O4 appears as a tan-colored solid that is insoluble in water, acids, or diluted alkali. Because of their high opacity, zinc ferrites can be used as pigments, especially in applications requiring heat stability. For example, zinc ferrite prepared from yellow iron oxide can be used as a substitute for applications in temperatures above . When added to high corrosion-resistant coatings, the corrosion protection increases with an increase in the concentration of zinc ferrite. One investigation shows that the zinc ferrite, which is paramagnetic in the bulk form, exhibits ferrimagnetism in nanocrystalline thin film format. A large r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
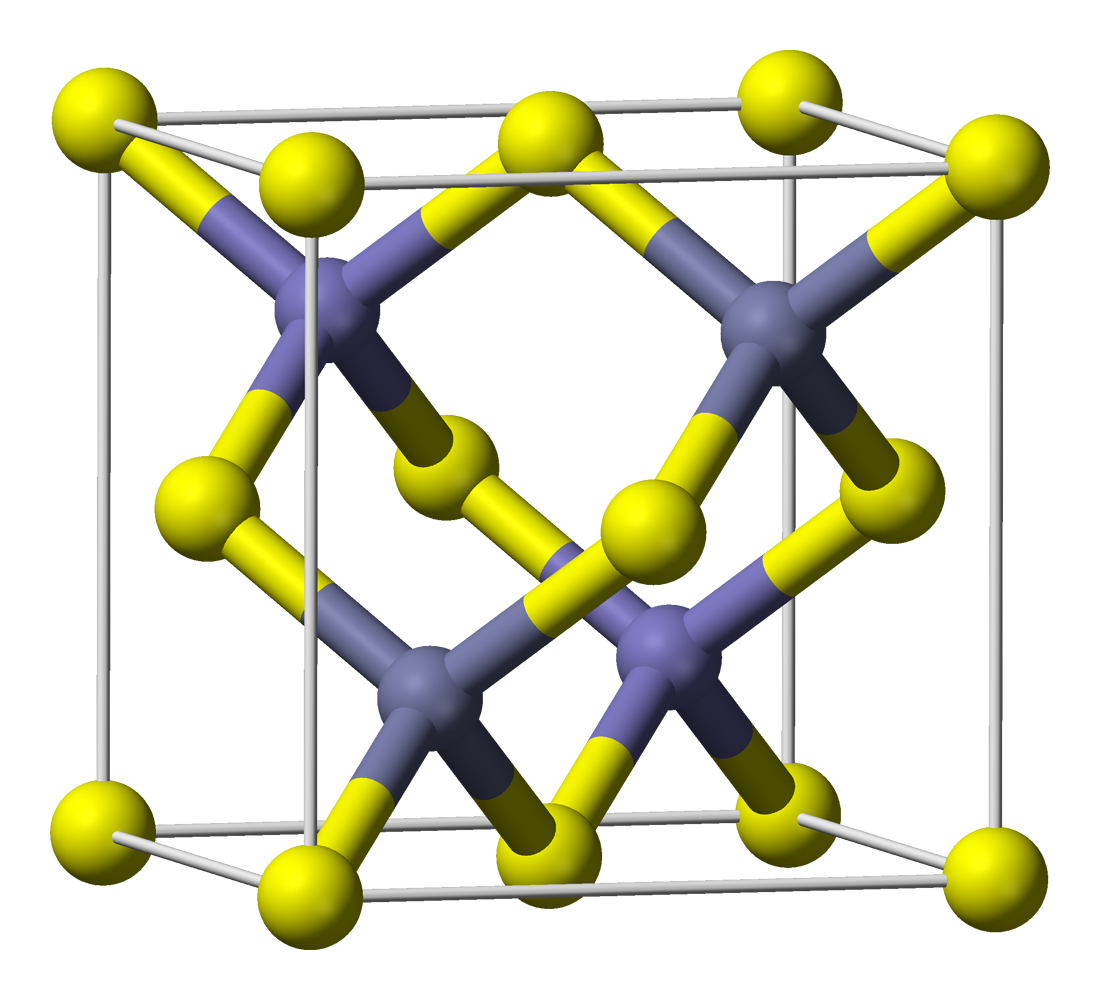
Zinc Sulphide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics. Structure ZnS exists in two main crystalline forms. This dualism is an example of polymorphism. In each form, the coordination geometry at Zn and S is tetrahedral. The more stable cubic form is known also as zinc blende or sphalerite. The hexagonal form is known as the mineral wurtzite, although it also can be produced synthetically.. The transition from the sphalerite form to the wurtzite form occurs at around 1020 °C. Applications Luminescent material left, 200 px, samples of zinc sulfide with varying sulfur vacancies. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanised
Galvanization ( also spelled galvanisation) is the process of applying a protective zinc coating to steel or iron, to prevent rusting. The most common method is hot-dip galvanizing, in which the parts are coated by submerging them in a bath of hot, molten zinc. Protective action The zinc coating, when intact, prevents corrosive substances from reaching the underlying iron. It's main function is to act as a sacrificial anode to prevent the iron from rusting by cathodic protection. Zinc is more reactive than iron, so the zinc coating preferentially oxidizes to zinc carbonate, preventing the iron from corroding, even if there are gaps in the zinc coating. Additional electroplating such as a chromate conversion coating may be applied to provide further surface passivation to the substrate material. History and etymology The process is named after the Italian physician, physicist, biologist and philosopher Luigi Galvani (9 September 1737 – 4 December 1798). The earliest ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Best Available Technology
The best available technology or best available techniques (BAT) is the technology approved by legislators or regulators for meeting output Technical standard, standards for a particular process, such as pollution abatement. Similar terms are ''best practicable means'' or ''best practicable environmental option''. BAT is a moving target on practices, since developing societal values and advancing techniques may change what is currently regarded as "reasonably achievable", "best practicable" and "best available". A literal understanding will connect it with a "spare no expense" doctrine which prescribes the acquisition of the best state of the art technology available, without regard for traditional cost-benefit analysis. In practical use, the cost aspect is also taken into account. See also discussions on the topic of the precautionary principle which, along with considerations of ''best available technologies'' and ''cost-benefit analyses'', is also involved in discussions leading ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |