|
Two-dimensional Nuclear Magnetic Resonance Spectroscopy
Two-Dimensional Nuclear Magnetic Resonance (2D NMR) is an advanced spectroscopic technique that builds upon the capabilities of one-dimensional (1D) NMR by incorporating an additional frequency dimension. This extension allows for a more comprehensive analysis of molecular structures. In 2D NMR, signals are distributed across two frequency axes, providing improved resolution and separation of overlapping peaks, particularly beneficial for studying complex molecules. This technique identifies correlations between different nuclei within a molecule, facilitating the determination of connectivity, spatial proximity, and dynamic interactions. 2D NMR encompasses a variety of experiments, including COSY (Correlation Spectroscopy), TOCSY (Total Correlation Spectroscopy), NOESY (Nuclear Overhauser Effect Spectroscopy), and HSQC (Heteronuclear Single Quantum Coherence). These techniques are indispensable in fields such as structural biology, where they are pivotal in determining protein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
NMR Instrument
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla (unit), tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. High-resolution nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
2-butanone
Butanone, also known as methyl ethyl ketone (MEK) or ethyl methyl ketone, is an organic compound with the formula CH3C(O)CH2CH3. This colorless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts.Wilhelm Neier, Guenter Strehlke "2-Butanone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran. Production Butanone may be produced by oxidation of 2-butanol. The dehydrogenation of 2-butanol is catalysed by copper, zinc, or bronze: :CH3CH(OH)CH2CH3 → CH3C(O)CH2CH3 + H2 This is used to produce approximately 700 million kilograms yearly. Other syntheses that have been examined but not implemented include Wacker oxidation of 2-butene and oxidation of isobutylbenzene, which is analogous to the industrial production of acet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Molecular Weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Rotational Correlation Time
Rotational correlation time (\tau_c) is the average time it takes for a molecule to rotate one radian. In solution, rotational correlation times are in the order of picoseconds. For example, the \tau_c = 1.7 ps for water, and 100 ps for a pyrroline nitroxyl radical in a DMSO-water mixture. Rotational correlation times are employed in the measurement of microviscosity (viscosity at the molecular level) and in protein characterization. Rotational correlation times may be measured by rotational (microwave), dielectric, and nuclear magnetic resonance (NMR) spectroscopy. Rotational correlation times of probe molecules in media have been measured by fluorescence lifetime or for radicals, from the linewidths of electron spin resonance Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spin ...s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Protein NMR
Nuclear magnetic resonance spectroscopy of proteins (usually abbreviated protein NMR) is a field of structural biology in which NMR spectroscopy is used to obtain information about the structure and dynamics of proteins, and also nucleic acids, and their complexes. The field was pioneered by Richard R. Ernst and Kurt Wüthrich at the ETH, and by Ad Bax, Marius Clore, Angela Gronenborn at the National Institutes of Health, NIH, and Gerhard Wagner (physicist), Gerhard Wagner at Harvard University, among others. Structure determination by NMR spectroscopy usually consists of several phases, each using a separate set of highly specialized techniques. The sample is prepared, measurements are made, interpretive approaches are applied, and a structure is calculated and validated. NMR involves the quantum-mechanical properties of the central core ("Atomic nucleus, nucleus") of the atom. These properties depend on the local molecular environment, and their measurement provides a map of how t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Spin–lattice Relaxation
During nuclear magnetic resonance observations, spin–lattice relaxation is the mechanism by which the longitudinal component of the total nuclear magnetic moment vector (parallel to the constant magnetic field) exponentially relaxes from a higher energy, non-equilibrium state to thermodynamic equilibrium with its surroundings (the "lattice"). It is characterized by the spin–lattice relaxation time, a time constant known as ''T1''. There is a different parameter, ''T2'', the spin–spin relaxation time, which concerns the exponential relaxation of the transverse component of the nuclear magnetization vector ( to the external magnetic field). Measuring the variation of ''T1'' and ''T2'' in different materials is the basis for some magnetic resonance imaging techniques. Nuclear physics ''T1'' relaxation or longitudinal relaxation curve ''T1'' characterizes the rate at which the longitudinal ''Mz'' component of the magnetization vector recovers exponentially towards its thermody ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nuclear Overhauser Effect
The nuclear Overhauser effect (NOE) is the transfer of spin polarization, nuclear spin polarization from one population of Spin (physics), spin-active nuclei (e.g. 1H, 13C, 15N etc.) to another via Relaxation (NMR), cross-relaxation. A phenomenological definition of the NOE in nuclear magnetic resonance spectroscopy (NMR) is the change in the integrated intensity (positive or negative) of one NMR resonance that occurs when another is saturated by irradiation with an Radio frequency, RF field. The change in resonance intensity of a nucleus is a consequence of the nucleus being close in space to those directly affected by the RF perturbation. The NOE is particularly important in the assignment of NMR resonances, and the elucidation and confirmation of the structures or configurations of organic and biological molecules. The 1H two-dimensional NOE spectroscopy (NOESY) experiment and its extensions are important tools to identify stereochemistry of proteins and other biomolecules in sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
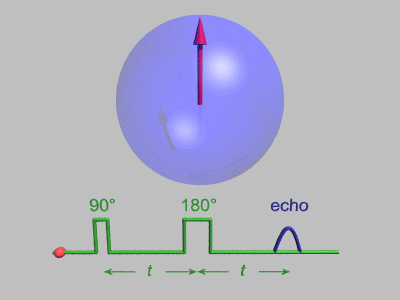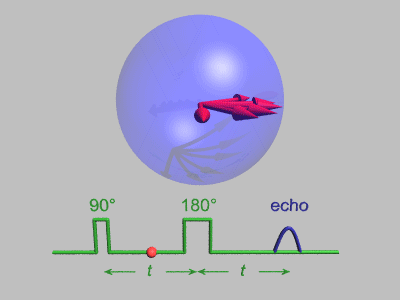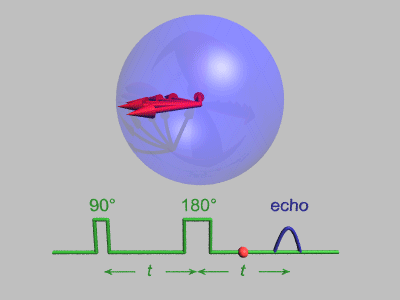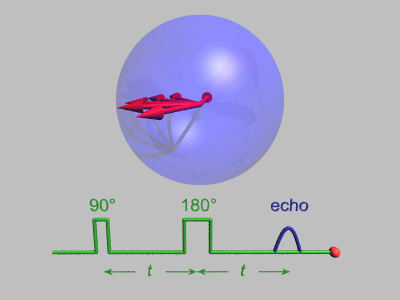
Spin Echo
In magnetic resonance, a spin echo or Hahn echo is the refocusing of spin magnetisation by a pulse of resonant electromagnetic radiation. Modern nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI) make use of this effect. The NMR signal observed following an initial excitation pulse decays with time due to both spin relaxation and any ''inhomogeneous'' effects which cause spins in the sample to precess at different rates. The first of these, relaxation, leads to an irreversible loss of magnetisation. But the inhomogeneous dephasing can be removed by applying a 180° ''inversion'' pulse that inverts the magnetisation vectors. Examples of inhomogeneous effects include a magnetic field gradient and a distribution of chemical shifts. If the inversion pulse is applied after a period ''t'' of dephasing, the inhomogeneous evolution will rephase to form an echo at time 2''t''. In simple cases, the intensity of the echo relative to the initial signal is given by '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Insensitive Nuclei Enhanced By Polarization Transfer
Insensitive nuclei enhancement by polarization transfer (INEPT) is a signal enhancement method used in NMR spectroscopy. It involves the transfer of nuclear spin polarization from spins with large Boltzmann population differences to nuclear spins of interest with lower Boltzmann population differences. INEPT uses J-coupling for the polarization transfer in contrast to Nuclear Overhauser effect (NOE), which arises from dipolar cross- relaxation. This method of signal enhancement was introduced by Ray Freeman in 1979. Due to its usefulness in signal enhancement, pulse sequences used in heteronuclear NMR experiments often contain blocks of INEPT or INEPT-like sequences. Background The sensitivity of NMR signal detection depends on the gyromagnetic ratio (γ) of the nucleus. In general, the signal intensity produced from a nucleus with a gyromagnetic ratio of γ is proportional to γ3 because the magnetic moment, the Boltzmann populations, and the nuclear precession frequency all in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Natural Abundance
In physics, natural abundance (NA) refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass (a weighted average, weighted by mole-fraction abundance figures) of these isotopes is the atomic weight listed for the element in the periodic table. The abundance of an isotope varies from planet to planet, and even from place to place on the Earth, but remains relatively constant in time (on a short-term scale). As an example, uranium has three naturally occurring isotopes: 238U, 235U, and 234U. Their respective natural mole-fraction abundances are 99.2739–99.2752%, 0.7198–0.7202%, and 0.0050–0.0059%. For example, if 100,000 uranium atoms were analyzed, one would expect to find approximately 99,274 238U atoms, approximately 720 235U atoms, and very few (most likely 5 or 6) 234U atoms. This is because 238U is much more stable than 235U or 234U, as the half-life of each isotope reveals: 4.468 billion years for 238U compared ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |