|
Translocon
The translocon (also known as a translocator or translocation channel) is a complex of proteins associated with the translocation of polypeptides across membranes. In eukaryotes the term translocon most commonly refers to the complex that transports nascent polypeptides with a targeting signal sequence into the interior (cisternal or lumenal) space of the endoplasmic reticulum (ER) from the cytosol. This translocation process requires the protein to cross a hydrophobic lipid bilayer. The same complex is also used to integrate nascent proteins into the membrane itself (membrane proteins). In prokaryotes, a similar protein complex transports polypeptides across the (inner) plasma membrane or integrates membrane proteins. In either case, the protein complex is formed from Sec proteins (Sec: secretory), with the hetero-trimeric Sec61 being the channel. In prokaryotes, the homologous channel complex is known as SecYEG. This article focuses on the cell's native translocons, but pathogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
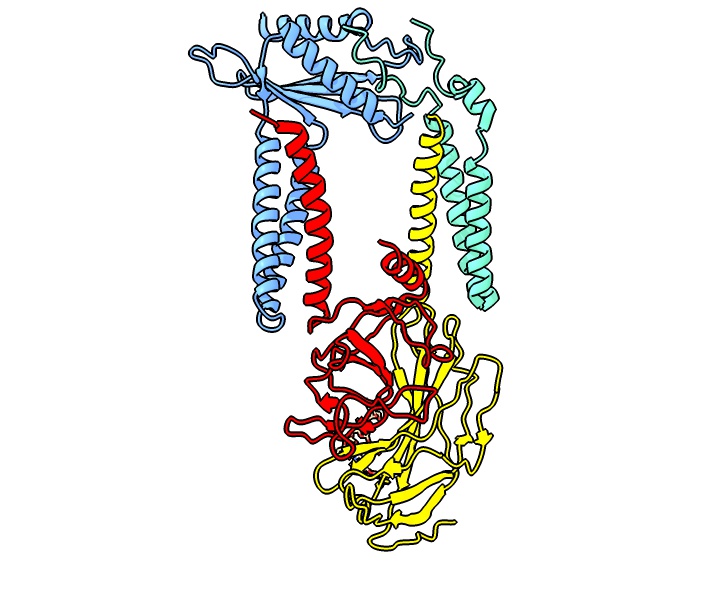
Sec61
Sec61, termed SecYEG in prokaryotes, is a membrane protein complex found in all domains of life. As the core component of the translocon, it transports proteins to the endoplasmic reticulum in eukaryotes and out of the cell in prokaryotes. It is a doughnut-shaped pore through the membrane with 3 different subunits (heterotrimeric), SecY (α), SecE (γ), and SecG (β). It has a region called the plug that blocks transport into or out of the ER. This plug is displaced when the hydrophobic region of a nascent polypeptide interacts with another region of Sec61 called the seam, allowing translocation of the polypeptide into the ER lumen. Although SecY and SecE are conserved in all three domains of life, bacterial SecG is only weakly homologous with eukaryotic Sec61β. The eukaryotic Sec61β is however homologous to the archaeal "SecG", leading some authors to refer to the archaeal complex as SecYEβ instead of SecYEG. (All three components of the archaeal complex are closer to their euka ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligosaccharyl Transferase
Oligosaccharyltransferase or OST () is a membrane protein complex that transfers a 14-sugar oligosaccharide from dolichol to nascent protein. It is a type of glycosyltransferase. The sugar Glc3Man9GlcNAc2 (where Glc=Glucose, Man=Mannose, and GlcNAc= ''N''-acetylglucosamine) is attached to an asparagine (Asn) residue in the sequence Asn-X- Ser or Asn-X- Thr where X is any amino acid except proline. This sequence is called a glycosylation ''sequon.'' The reaction catalyzed by OST is the central step in the ''N''-linked glycosylation pathway. Location OST is a component of the translocon in the endoplasmic reticulum (ER) membrane. A lipid-linked core-oligosaccharide is assembled at the membrane of the endoplasmic reticulum and transferred to selected asparagine residues of nascent polypeptide chains by the oligosaccharyl transferase complex. The active site of OST is located about 4 nm from the lumenal face of the ER membrane. It usually acts during translation as the nas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Targeting
Protein targeting or protein sorting is the biological mechanism by which proteins are transported to their appropriate destinations within or outside the cell. Proteins can be targeted to the inner space of an organelle, different intracellular membranes, the plasma membrane, or to the exterior of the cell via secretion. Information contained in the protein itself directs this delivery process. Correct sorting is crucial for the cell; errors or dysfunction in sorting have been linked to multiple diseases. History In 1970, Günter Blobel conducted experiments on protein translocation across membranes. Blobel, then an assistant professor at Rockefeller University, built upon the work of his colleague George Palade. Palade had previously demonstrated that non-secreted proteins were translated by free ribosomes in the cytosol, while secreted proteins (and target proteins, in general) were translated by ribosomes bound to the endoplasmic reticulum (ER). Candidate explanations at t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryote, eukaryotic cell, and has many other important functions such as protein folding. The word endoplasmic means "within the cytoplasm", and reticulum is Latin for "little net". It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. There are two types of ER that share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of Cell (biology), cells contain different ratios of the two types of ER dependin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Signal-recognition Particle
The signal recognition particle (SRP) is an abundant, cytosolic, universally conserved ribonucleoprotein (protein-RNA complex) that recognizes and targets specific proteins to the endoplasmic reticulum in eukaryotes and the plasma membrane in prokaryotes. History The function of SRP was discovered by the study of processed and unprocessed secretory proteins, particularly immunoglobulin light chains; and bovine preprolactin. Newly synthesized proteins in eukaryotes carry N-terminal hydrophobic signal sequences, which are bound by SRP when they emerge from the ribosome. Mechanism In eukaryotes, SRP binds to the signal sequence of a newly synthesized peptide as it emerges from the ribosome. This binding leads to the slowing of protein synthesis known as "elongation arrest", a conserved function of SRP that facilitates the coupling of the protein translation and the protein translocation processes. SRP then targets this entire complex (the ribosome-nascent chain complex) to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Immunoglobulin Protein
Binding immunoglobulin protein (BiPS) also known as 78 kDa glucose-regulated protein (GRP-78) or heat shock 70 kDa protein 5 (HSPA5) is a protein that in humans is encoded by the ''HSPA5'' gene. BiP is a HSP70 molecular chaperone located in the lumen of the endoplasmic reticulum (ER) that binds newly synthesized proteins as they are translocated into the ER, and maintains them in a state competent for subsequent folding and oligomerization. BiP is also an essential component of the translocation machinery and plays a role in retrograde transport across the ER membrane of aberrant proteins destined for degradation by the proteasome. BiP is an abundant protein under all growth conditions, but its synthesis is markedly induced under conditions that lead to the accumulation of unfolded polypeptides in the ER. Structure BiP contains two functional domains: a nucleotide-binding domain (NBD) and a substrate-binding domain (SBD). The NBD binds and hydrolyzes ATP, and the SBD binds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SRP Receptor
Signal recognition particle (SRP) receptor, also called the docking protein, is a dimer composed of 2 different subunits that are associated exclusively with the rough ER in mammalian cells. Its main function is to identify the SRP units. SRP (signal recognition particle) is a molecule that helps the ribosome-mRNA-polypeptide complexes to settle down on the membrane of the endoplasmic reticulum. The eukaryotic SRP receptor (termed SR) is a heterodimer of SR-alpha (70 kDa; SRPRA) and SR-beta (25 kDa; SRPRB), both of which contain a GTP-binding domain, while the prokaryotic SRP receptor comprises only the monomeric loosely membrane-associated SR-alpha homologue FtsY (). SRX domain SR-alpha regulates the targeting of SRP-ribosome-nascent polypeptide complexes to the translocon. SR-alpha binds to the SRP54 subunit of the SRP complex. The SR-beta subunit is a transmembrane GTPase that anchors the SR-alpha subunit (a peripheral membrane GTPase) to the ER membrane. SR-beta interac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Signal Peptidase
Signal peptidases are enzymes that convert secretory and some membrane proteins to their mature or pro forms by cleaving their signal peptides from their N-termini. Signal peptidases were initially observed in endoplasmic reticulum (ER)-derived membrane fractions isolated from mouse myeloma cells. The key observation by César Milstein and colleagues was that immunoglobulin light chains were produced in a higher molecular weight form, which became processed by the ER membrane fraction. This finding was directly followed by the discovery of the translocation machinery. Signal peptidases are also found in prokaryotes A prokaryote (; less commonly spelled procaryote) is a single-celled organism whose cell lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Ancient Greek (), meaning 'before', and (), meaning 'nut' ... as well as the protein import machinery of mitochondria and chloroplasts. All signal peptidases described s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Helix
A transmembrane domain (TMD, TM domain) is a membrane-spanning protein domain. TMDs may consist of one or several alpha-helices or a transmembrane beta barrel. Because the interior of the lipid bilayer is hydrophobic, the amino acid residues in TMDs are often hydrophobic, although proteins such as membrane pumps and ion channels can contain polar residues. TMDs vary greatly in size and hydrophobicity; they may adopt organelle-specific properties. Functions of transmembrane domains Transmembrane domains are known to perform a variety of functions. These include: * Anchoring transmembrane proteins to the membrane. *Facilitating molecular transport of molecules such as ions and proteins across biological membranes; usually hydrophilic residues and binding sites in the TMDs help in this process. *Signal transduction across the membrane; many transmembrane proteins, such as G protein-coupled receptors, receive extracellular signals. TMDs then propagate those signals across the mem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Translation (biology)
In biology, translation is the process in living Cell (biology), cells in which proteins are produced using RNA molecules as templates. The generated protein is a sequence of amino acids. This sequence is determined by the sequence of nucleotides in the RNA. The nucleotides are considered three at a time. Each such triple results in the addition of one specific amino acid to the protein being generated. The matching from nucleotide triple to amino acid is called the genetic code. The translation is performed by a large complex of functional RNA and proteins called ribosomes. The entire process is called gene expression. In translation, messenger RNA (mRNA) is decoded in a ribosome, outside the nucleus, to produce a specific amino acid chain, or polypeptide. The polypeptide later protein folding, folds into an Activation energy, active protein and performs its functions in the cell. The polypeptide can also start folding during protein synthesis. The ribosome facilitates decoding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SEC62
Translocation protein SEC62 is a protein that in humans is encoded by the ''SEC62'' gene. Function The Sec61 complex is the central component of the protein translocation apparatus of the endoplasmic reticulum (ER) membrane. The protein encoded by this gene and SEC63 protein are found to be associated with ribosome-free SEC61 complex. It is speculated that Sec61-Sec62-Sec63 may perform post-translational protein translocation into the ER. The Sec61-Sec62-Sec63 complex might also perform the backward transport of ER proteins that are subject to the ubiquitin-proteasome-dependent degradation pathway. The encoded protein is an integral membrane protein An integral, or intrinsic, membrane protein (IMP) is a type of membrane protein that is permanently attached to the biological membrane. All transmembrane proteins can be classified as IMPs, but not all IMPs are transmembrane proteins. IMPs comp ... located in the rough ER. References Further reading * * * * * * * {{g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |