|
Sugar Alcohol
Sugar alcohols (also called polyhydric alcohols, polyalcohols, alditols or glycitols) are organic compounds, typically derived from sugars, containing one hydroxyl group attached to each carbon atom. They are white, water-soluble solids that can occur naturally or be produced industrially by hydrogenating sugars. Since they contain multiple groups, they are classified as polyols. Sugar alcohols are used widely in the food industry as thickeners and sweeteners. In commercial foodstuffs, sugar alcohols are commonly used in place of table sugar (sucrose), often in combination with high-intensity artificial sweeteners, in order to offset their low sweetness. Xylitol and sorbitol are popular sugar alcohols in commercial foods. Structure Sugar alcohols have the general formula . In contrast, sugars have two fewer hydrogen atoms, for example, or . Like their parent sugars, sugar alcohols exist in diverse chain length. Most have five- or six-carbon chains, because they are deriv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Erythritol Structure
Erythritol (, ) is an organic compound, the naturally occurring achiral meso four-carbon sugar alcohol (or polyol). It is the reduced form of either D- or L- erythrose and one of the two reduced forms of erythrulose. It is used as a food additive and sugar substitute. It is synthesized from corn using enzymes and fermentation. Its formula is , or HO(CH2)(CHOH)2(CH2)OH. Erythritol is 60–70% as sweet as table sugar. However, erythritol is almost completely noncaloric and does not affect blood sugar or cause tooth decay. Japanese companies pioneered the commercial development of erythritol as a sweetener in the 1990s. Etymology The name "erythritol" derives from the Greek word for the color red (''erythros'' or ). That is the case even though erythritol is almost always found in the form of white crystals or powder, and chemical reactions do not turn it red. The name "erythritol" is adapted from a closely-related compound, erythrin, which turns red upon oxidation. Histor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets, and is contained in large amounts in staple foods such as wheat, potatoes, maize (corn), rice, and cassava (manioc). Pure starch is a white, tasteless and odorless powder that is insoluble in cold water or Alcohol (chemistry), alcohol. It consists of two types of molecules: the linear and helix, helical amylose and the branched amylopectin. Depending on the plant, starch generally contains 20 to 25% amylose and 75 to 80% amylopectin by weight. Glycogen, the energy reserve of animals, is a more highly branched version of amylopectin. In industry, starch is often converted into sugars, for example by malting. These sugars may be fermentation, fermented to produce ethanol in the manufacture of beer, whisky and biofuel. In addition, sugars ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
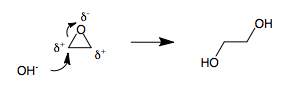
Ethylene Glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. It has a sweet taste but is toxic in high concentrations. This molecule has been observed in outer space. Production Industrial routes Ethylene glycol is produced from ethylene (ethene), via the intermediate ethylene oxide. Ethylene oxide reacts with water to produce ethylene glycol according to the chemical equation : This reaction can be catalyzed by either acids or bases or can occur at neutral pH under elevated temperatures. The highest yields of ethylene glycol occur at acidic or neutral pH with a large excess of water. Under these conditions, ethylene glycol yields of 90% can be achieved. The major byproducts are the oligomers diethylene glycol, triethylene glycol, and tetra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycemic Index
The glycemic (glycaemic) index (GI; ) is a number from 0 to 100 assigned to a food, with pure glucose arbitrarily given the value of 100, which represents the relative rise in the blood glucose level two hours after consuming that food. The GI of a specific food depends primarily on the type of carbohydrate it contains, but is also affected by the amount of entrapment of the carbohydrate molecules within the food, the fat, protein content of the food, the moisture and fiber content, the amount of organic acids (or their salts) (e.g., citric or acetic acid), and the method of cooking. GI tables, which list many types of foods and their GIs, are available. A food is considered to have a ''low GI'' if it is 55 or less; ''high GI'' if 70 or more; and ''mid-range GI'' if 56 to 69. The term was introduced in 1981 by David J. Jenkins and co-workers and was created to compare the relative effects of different foods on postprandial glucose levels. It is useful for quantifying the relati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Sugar
The blood sugar level, blood sugar concentration, blood glucose level, or glycemia is the measure of glucose concentrated in the blood. The body tightly regulates blood glucose levels as a part of metabolic homeostasis. For a 70 kg (154 lb) human, approximately four grams of dissolved glucose (also called "blood glucose") is maintained in the blood plasma at all times. Glucose that is not circulating in the blood is stored in skeletal muscle and liver cells in the form of glycogen; in fasting individuals, blood glucose is maintained at a constant level by releasing just enough glucose from these glycogen stores in the liver and skeletal muscle in order to maintain homeostasis. Glucose can be transported from the intestines or liver to other tissues in the body via the bloodstream. Cellular glucose uptake is primarily regulated by insulin, a hormone produced in the pancreas. Once inside the cell, the glucose can now act as an energy source as it undergoes the pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tooth Decay
Tooth decay, also known as caries,The word 'caries' is a mass noun, and is not a plural of 'carie'.'' is the breakdown of teeth due to acids produced by bacteria. The resulting cavities may be a number of different colors, from yellow to black. Symptoms may include pain and difficulty eating. Complications may include periodontal disease, inflammation of the tissue around the tooth, tooth loss and infection or dental abscess, abscess formation. Tooth regeneration is an ongoing Stem-cell therapy, stem cell–based field of study that aims to find methods to reverse the effects of decay; current methods are based on easing symptoms. The cause of cavities is acid from bacteria dissolving the hard tissues of the teeth (Tooth enamel, enamel, dentin and cementum). The acid is produced by the bacteria when they break down food debris or sugar on the tooth surface. Simple sugars in food are these bacteria's primary energy source and thus a diet high in simple sugar is a risk factor. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermentation
Fermentation is a type of anaerobic metabolism which harnesses the redox potential of the reactants to make adenosine triphosphate (ATP) and organic end products. Organic molecules, such as glucose or other sugars, are catabolized and reduced by donating their electrons to other organic molecules (cofactors, coenzymes, etc.). Fermentation is important in several areas of human society. Humans have used fermentation in the production and preservation of food for 13,000 years. It has been associated with health benefits, unique flavor profiles, and making products have better texture. Humans and their livestock also benefit from fermentation from the microbes in the gut that release end products that are subsequently used by the host for energy. Perhaps the most commonly known use for fermentation is at an industrial level to produce commodity chemicals, such as ethanol and lactate. Ethanol is used in a variety of alcoholic beverages (beers, wine, and spirits) while lactate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Erythritol
Erythritol (, ) is an organic compound, the naturally occurring achiral meso four-carbon sugar alcohol (or polyol). It is the reduced form of either D- or L- erythrose and one of the two reduced forms of erythrulose. It is used as a food additive and sugar substitute. It is synthesized from corn using enzymes and fermentation. Its formula is , or HO(CH2)(CHOH)2(CH2)OH. Erythritol is 60–70% as sweet as table sugar. However, erythritol is almost completely noncaloric and does not affect blood sugar or cause tooth decay. Japanese companies pioneered the commercial development of erythritol as a sweetener in the 1990s. Etymology The name "erythritol" derives from the Greek word for the color red (''erythros'' or ). That is the case even though erythritol is almost always found in the form of white crystals or powder, and chemical reactions do not turn it red. The name "erythritol" is adapted from a closely-related compound, erythrin, which turns red upon oxidation. Hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Raney Nickel
Raney nickel , also called spongy nickel, is a fine-grained solid composed mostly of nickel derived from a nickel–aluminium alloy. Several grades are known, of which most are gray solids. Some are pyrophoric, but most are used as air-stable slurries. Raney nickel is used as a reagent and as a catalyst in organic chemistry. It was developed in 1926 by American engineer Murray Raney for the hydrogenation of vegetable oils. Raney Nickel is a registered trademark of W. R. Grace and Company. Other major producers are Evonik and Johnson Matthey. Preparation Alloy preparation The Ni–Al alloy is prepared by dissolving nickel in molten aluminium followed by cooling ("quenching"). Depending on the Ni:Al ratio, quenching produces a number of different phases. During the quenching procedure, small amounts of a third metal, such as zinc or chromium, are added to enhance the activity of the resulting catalyst. This third metal is called a "Promoter (catalysis), promoter". The promoter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mannitol
Mannitol is a type of sugar alcohol used as a sweetener and medication. It is used as a low calorie sweetener as it is poorly absorbed by the intestines. As a medication, it is used to decrease pressure in the eyes, as in glaucoma, and to lower increased intracranial pressure. Medically, it is given by injection or inhalation. Effects typically begin within 15 minutes and last up to 8 hours. Common side effects from medical use include electrolyte problems and dehydration. Other serious side effects may include worsening heart failure and kidney problems. It is unclear if use is safe in pregnancy. Mannitol is in the osmotic diuretic family of medications and works by pulling fluid from the brain and eyes. The discovery of mannitol is attributed to Joseph Louis Proust in 1806. It is on the World Health Organization's List of Essential Medicines. It was originally made from the flowering ash and called manna due to its supposed resemblance to the Biblical food. Man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same cataly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Cleavage
In chemistry, bond cleavage, or bond fission, is the splitting of chemical bonds. This can be generally referred to as dissociation when a molecule is cleaved into two or more fragments. In general, there are two classifications for bond cleavage: ''homo''lytic and ''hetero''lytic, depending on the nature of the process. The triplet and singlet excitation energies of a sigma bond can be used to determine if a bond will follow the homolytic or heterolytic pathway. A metal−metal sigma bond is an exception because the bond's excitation energy is extremely high, thus cannot be used for observation purposes. In some cases, bond cleavage requires catalysts. Due to the high bond-dissociation energy of C−H bonds, around , a large amount of energy is required to cleave the hydrogen atom from the carbon and bond a different atom to the carbon. Homolytic cleavage In homolytic cleavage, or homolysis, the two electrons in a cleaved covalent bond are divided equally betwee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |