|
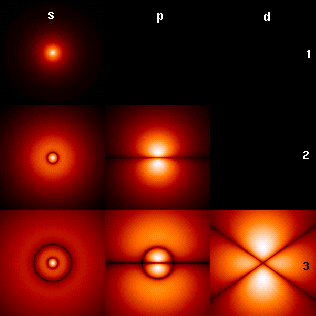
Spectroscopic Notation
Spectroscopic notation provides a way to specify atomic ionization states, atomic orbitals, and molecular orbitals. Ionization states Spectroscopists customarily refer to the spectrum arising from a given ionization state of a given element by the element's symbol followed by a Roman numeral. The numeral I is used for spectral lines associated with the neutral element, II for those from the first ionization state, III for those from the second ionization state, and so on. For example, "He I" denotes lines of neutral helium, and "C IV" denotes lines arising from the third ionization state, C3+, of carbon. This notation is used for example to retrieve data from thNIST Atomic Spectrum Database Atomic and molecular orbitals Before atomic orbitals were understood, spectroscopists discovered various distinctive series of spectral lines in atomic spectra, which they identified by letters. These letters were later associated with the azimuthal quantum number, ''ℓ''. The let ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Russel–Saunders Term Symbol
In atomic physics, a term symbol is an abbreviated description of the total spin and orbital angular momentum quantum numbers of the electrons in a multi-electron atom. So while the word ''symbol'' suggests otherwise, it represents an actual ''value'' of a physical quantity. For a given electron configuration of an atom, its state depends also on its total angular momentum, including spin and orbital components, which are specified by the term symbol. The usual atomic term symbols assume LS coupling (also known as Russell–Saunders coupling) in which the all-electron total quantum numbers for orbital (''L''), spin (''S'') and total (''J'') angular momenta are good quantum numbers. In the terminology of atomic spectroscopy, ''L'' and ''S'' together specify a term; ''L'', ''S'', and ''J'' specify a level; and ''L'', ''S'', ''J'' and the magnetic quantum number ''M''''J'' specify a state. The conventional term symbol has the form 2''S''+1''L''''J'', where ''J'' is written optiona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Symmetry
In chemistry, molecular symmetry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explain many of a molecule's chemical property, chemical properties, such as whether or not it has a molecular dipole moment, dipole moment, as well as its allowed spectroscopy, spectroscopic transitions. To do this it is necessary to use group theory. This involves classifying the states of the molecule using the irreducible representations from the character table of the symmetry group of the molecule. Symmetry is useful in the study of molecular orbitals, with applications to the Hückel method, to ligand field theory, and to the Woodward–Hoffmann rules. Many university level textbooks on physical chemistry, quantum chemistry, spectroscopy and inorganic chemistry discuss symmetry. Another framework on a larger scale is the use of crystal sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Notation
X-ray notation is a method of labeling atomic orbitals that grew out of X-ray science. Also known as IUPAC notation, it was adopted by the International Union of Pure and Applied Chemistry in 1991 as a simplification of the older Siegbahn notation. In X-ray notation, every principal quantum number is given a letter associated with it. In many areas of physics and chemistry, atomic orbitals are described with spectroscopic notation (1s, 2s, 2p, 3s, 3p, etc.), but the more traditional X-ray notation is still used with most X-ray spectroscopy techniques including AES and XPS. Conversion Uses *X-ray sources are classified by the type of material and orbital used to generate them. For example, CuKα X-rays are emitted from the K orbital of copper. *X-ray absorption is reported as which orbital absorbed the x-ray photon. In EXAFS and XMCD the L-edge or the L absorption edge is the point where the L orbital begins to absorb x-rays. * Auger peaks are identified with three orbital de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Term Symbol
In atomic physics, a term symbol is an abbreviated description of the total spin and orbital angular momentum quantum numbers of the electrons in a multi-electron atom. So while the word ''symbol'' suggests otherwise, it represents an actual ''value'' of a physical quantity. For a given electron configuration of an atom, its state depends also on its total angular momentum, including spin and orbital components, which are specified by the term symbol. The usual atomic term symbols assume angular momentum coupling#LS coupling, LS coupling (also known as Russell–Saunders coupling) in which the all-electron total quantum numbers for orbital (''L''), spin (''S'') and total (''J'') angular momenta are good quantum numbers. In the terminology of atomic spectroscopy, ''L'' and ''S'' together specify a term; ''L'', ''S'', and ''J'' specify a level; and ''L'', ''S'', ''J'' and the magnetic quantum number ''M''''J'' specify a state. The conventional term symbol has the form 2''S''+1''L'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Principal Quantum Number
In quantum mechanics, the principal quantum number (''n'') of an electron in an atom indicates which electron shell or energy level it is in. Its values are natural numbers (1, 2, 3, ...). Hydrogen and Helium, at their lowest energies, have just one electron shell. Lithium through Neon (see periodic table) have two shells: two electrons in the first shell, and up to 8 in the second shell. Larger atoms have more shells. The principal quantum number is one of four quantum numbers assigned to each electron in an atom to describe the quantum state of the electron. The other quantum numbers for bound electrons are the total angular momentum of the orbit ''ℓ'', the angular momentum in the z direction ''ℓz'', and the spin of the electron ''s''. Overview and history As ''n'' increases, the electron is also at a higher energy and is, therefore, less tightly bound to the nucleus. For higher ''n'', the electron is farther from the nucleus, on average. For each value of ''n'', th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Chemistry Mnemonics
A mnemonic is a memory aid used to improve long-term memory and make the process of Memory consolidation, consolidation easier. Many chemistry aspects, rules, names of compounds, sequences of elements, their reactivity, etc., can be easily and efficiently memorized with the help of mnemonics. This article contains the list of certain mnemonics in chemistry. Orbitals Sequence of orbitals *''Sober Physicists Don't Find Giraffes Hiding In Kitchens.'' Note: After the k shell, they follow alphabetical order (skipping s and p as they came earlier). Aufbau principle The order of sequence of atomic orbitals (according to Madelung rule, Madelung rule or Klechkowski rule) can be remembered by the following. Periodic table Periods Periods 1, 2 and 3 : *''Hi Hello Little Beer Bottles Crack Nicely On Freddie's kNee. Nasties Merge All Silly People Suffer Clouts Arnti *''Happy Henry Likes Beans Brownies and Chocolate Nuts Over Friday's News. Naughty Margaret Always Sighs, "Ple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is , meaning that the 1s, 2s, and 2p subshells are occupied by two, two, and six electrons, respectively. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by the nuclei and all the other electrons. Mathematically, configurations are described by Slater determinants or configuration state functions. According to the laws of quantum mechanics, a level of energy is associated with each electron configuration. In certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon. Knowledge of the electron configuration of different atoms is useful in understanding the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azimuthal Quantum Number
In quantum mechanics, the azimuthal quantum number is a quantum number for an atomic orbital that determines its angular momentum operator, orbital angular momentum and describes aspects of the angular shape of the orbital. The azimuthal quantum number is the second of a set of quantum numbers that describe the unique quantum state of an electron (the others being the principal quantum number , the magnetic quantum number , and the spin quantum number ). For a given value of the principal quantum number (''electron shell''), the possible values of are the integers from 0 to . For instance, the shell has only orbitals with \ell=0, and the shell has only orbitals with \ell=0, and \ell=1. For a given value of the azimuthal quantum number , the possible values of the magnetic quantum number are the integers from to , including 0. In addition, the spin quantum number can take two distinct values. The set of orbitals associated with a particular value of are som ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Positronium
Positronium (Ps) is a system consisting of an electron and its antimatter, anti-particle, a positron, bound together into an exotic atom, specifically an onium. Unlike hydrogen, the system has no protons. The system is unstable: the two particles annihilate each other to predominantly produce two or three gamma-rays, depending on the relative spin states. The energy levels of the two particles are similar to that of the hydrogen atom (which is a bound state of a proton and an electron). However, because of the reduced mass, the frequency, frequencies of the spectral lines are less than half of those for the corresponding hydrogen lines. States The mass of positronium is 1.022 MeV, which is twice the electron mass minus the binding energy of a few eV. The lowest energy orbital state of positronium is 1S, and like with hydrogen, it has a hyperfine structure arising from the relative orientations of the spins of the electron and the positron. The Singlet state, ''singlet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quarkonium
In particle physics, quarkonium (from quark and -onium, pl. quarkonia) is a flavor (physics), flavorless meson whose constituents are a heavy quark and its own antiquark, making it both a neutral particle and its own antiparticle. The name "quarkonium" is analogous to positronium, the bound state of electron and Positron, anti-electron. The particles are short-lived due to matter-antimatter annihilation. Light quarks Light quarks (up quark, up, down quark, down, and strange quark, strange) are much less massive than the heavier quarks, and so the physical states actually seen in experiments (Eta meson, η, Eta meson, η′, and Pion, π0 mesons) are quantum mechanical mixtures of the light quark states. The much larger mass differences between the charm quark, charm and bottom quark, bottom quarks and the lighter quarks results in states that are well defined in terms of a quark–antiquark pair of a given flavor. Heavy quarks Quarkonia, bound state of ''charmonium'' (c\bar) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quark
A quark () is a type of elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nucleus, atomic nuclei. All commonly observable matter is composed of up quarks, down quarks and electrons. Owing to a phenomenon known as ''color confinement'', quarks are never found in isolation; they can be found only within hadrons, which include baryons (such as protons and neutrons) and mesons, or in quark–gluon plasmas. There is also the theoretical possibility of #Other_phases_of_quark_matter, more exotic phases of quark matter. For this reason, much of what is known about quarks has been drawn from observations of hadrons. Quarks have various Intrinsic and extrinsic properties, intrinsic physical property, properties, including electric charge, mass, color charge, and Spin (physics), spin. They are the only elementary particles in the Standard Mode ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |