|
Silver(I,III) Oxide
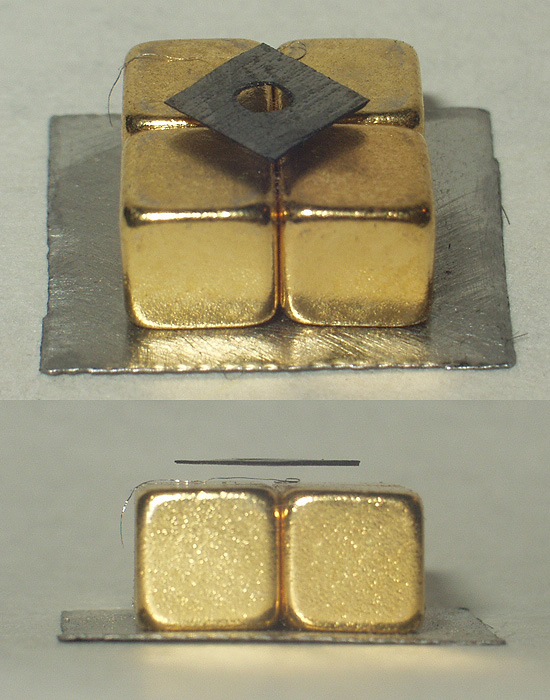
Silver(I,III) oxide or tetrasilver tetroxide is the inorganic compound with the formula Ag4O4. It is a component of silver zinc batteries. It can be prepared by the slow addition of a silver(I) salt to a persulfate solution e.g. AgNO3 to a Na2S2O8 solution. It adopts an unusual structure, being a mixed-valence compound. It is a dark brown solid that decomposes with evolution of O2 in water. It dissolves in concentrated nitric acid to give brown solutions containing the Ag2+ ion. Structure Although its empirical formula, AgO, suggests that the compound tetrasilver tetraoxide has silver in the +2 oxidation state, each unit has two monovalent silver atoms bonded to an oxygen atom, and two trivalent silver atoms bonded to three oxygen atoms, and it is in fact diamagnetic. X-ray diffraction studies show that the silver atoms adopt two different coordination environments, one having two collinear oxide neighbours and the other four coplanar oxide neighbours.Wells A.F. (1984) ''St ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamagnetic
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted by a magnetic field. Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In paramagnetic and ferromagnetic substances, the weak diamagnetic force is overcome by the attractive force of magnetic dipoles in the material. The magnetic permeability of diamagnetic materials is less than the permeability of vacuum, ''μ''0. In most materials, diamagnetism is a weak effect which can be detected only by sensitive laboratory instruments, but a superconductor acts as a strong diamagnet because it entirely expels any magnetic field from its interior (the Meissner effect). Diamagnetism was first discovered whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as synthetic dyes and medicines (e.g. metronidazole). Nitric acid is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Compounds
Silver is a relatively unreactive metal, although it can form several compounds. The common oxidation states of silver are (in order of commonness): +1 (the most stable state; for example, silver nitrate, AgNO3); +2 (highly oxidising; for example, silver(II) fluoride, AgF2); and even very rarely +3 (extreme oxidising; for example, potassium tetrafluoroargentate(III), KAgF4). The +3 state requires very strong oxidising agents to attain, such as fluorine or peroxodisulfate, and some silver(III) compounds react with atmospheric moisture and attack glass.Greenwood and Earnshaw, p. 1188 Indeed, silver(III) fluoride is usually obtained by reacting silver or silver monofluoride with the strongest known oxidizing agent, krypton difluoride.Greenwood and Earnshaw, p. 903 Oxides and chalcogenides Oxides Silver and gold have rather low chemical affinity, chemical affinities for oxygen, lower than copper, and it is therefore expected that silver oxides are thermally quite unstable. Soluble sil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herpes
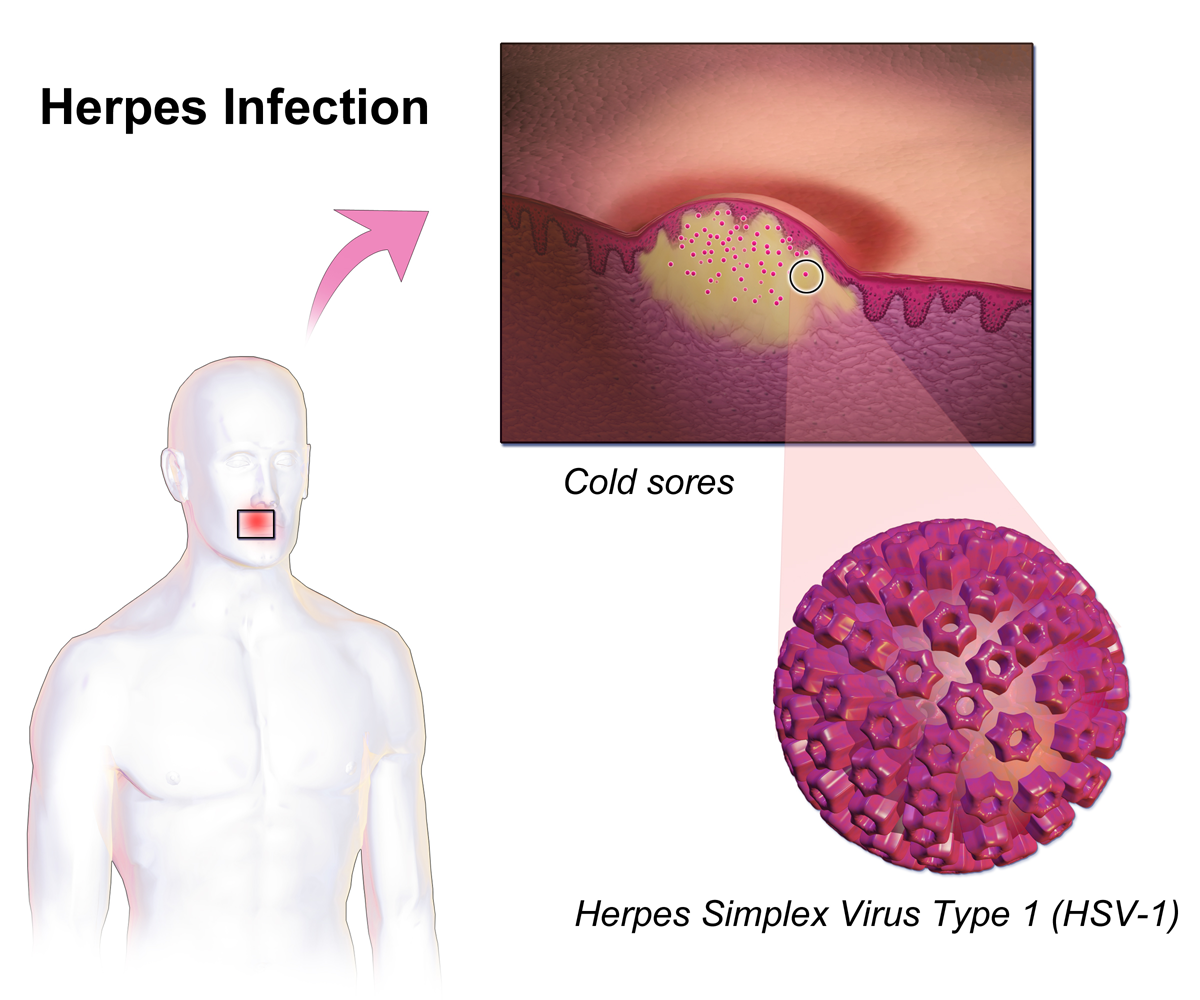
Herpes simplex, often known simply as herpes, is a viral infection caused by the herpes simplex virus. Herpes infections are categorized by the area of the body that is infected. The two major types of herpes are oral herpes and genital herpes, though other forms also exist. Oral herpes involves the face or mouth. It may result in small blisters in groups, often called cold sores or fever blisters, or may just cause a sore throat. Genital herpes involves the genitalia. It may have minimal symptoms or form blisters that break open and result in small ulcers. These typically heal over two to four weeks. Tingling or shooting pains may occur before the blisters appear. Herpes cycles between periods of active disease followed by periods without symptoms. The first episode is often more severe and may be associated with fever, muscle pains, swollen lymph nodes and headaches. Over time, episodes of active disease decrease in frequency and severity. Herpetic whitlow typically inv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxide
In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined to each other and to adjacent elements through Single bond, single covalent bonds, denoted by dashes or lines. The group in a peroxide is often called the peroxide group, though some nomenclature discrepancies exist. This linkage is recognized as a common polyatomic ion, and exists in many molecules. General structure The characteristic structure of any regular peroxide is the oxygen–oxygen covalent single bond, which connects the two main atoms together. In the event that the molecule has no chemical Substituent, substituents, the peroxide group will have a [−2] Formal charge, net charge. Each oxygen atom has a charge of negative one, as 5 of its Valence electron, valence electrons remain in the outermost Atomic orbital, orbital ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Diffraction
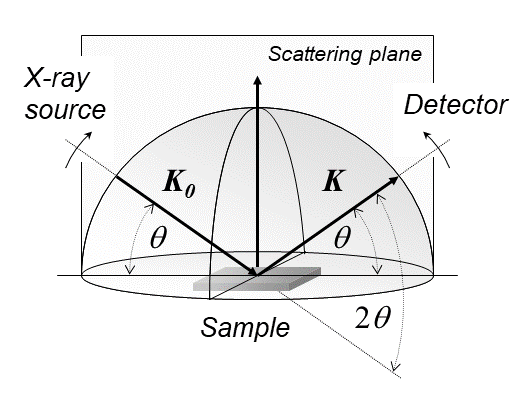
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. The resulting map of the directions of the X-rays far from the sample is called a diffraction pattern. It is different from X-ray crystallography which exploits X-ray diffraction to determine the arrangement of atoms in materials, and also has other components such as ways to map from experimental diffraction measurements to the positions of atoms. This article provides an overview of X-ray diffraction, starting with the early #History, history of x-rays and the discovery that they have the right spacings to be diffracted by crystals. In many cases these diffraction patterns can be #Introduction to x-ray diffraction theory, Interpreted using a single scattering or kinematical theory with conservation of energy (#Ewald's sphere, wave vecto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamagnetism
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted by a magnetic field. Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In paramagnetic and ferromagnetic substances, the weak diamagnetic force is overcome by the attractive force of magnetic dipoles in the material. The Permeability (electromagnetism), magnetic permeability of diamagnetic materials is less than the Vacuum_permeability, permeability of vacuum, ''μ''0. In most materials, diamagnetism is a weak effect which can be detected only by sensitive laboratory instruments, but a superconductivity, superconductor acts as a strong diamagnet because it entirely expels any magnetic field from its inter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation State
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. Beside nearly-pure ionic bonding, many covalent bonds exhibit a strong ionicity, making oxidation state a useful predictor of charge. The oxidation state of an atom does not represent the "real" charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the ionization energy required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on Electronegativities of the elements (data page), the choice of electronegativity scale used in their calculation. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Empirical Formula
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, is simply SO, as is the empirical formula of disulfur dioxide, S2O2. Thus, sulfur monoxide and disulfur dioxide, both compounds of sulfur and oxygen, have the same empirical formula. However, their molecular formulas, which express the number of atoms in each molecule of a chemical compound, are not the same. An empirical formula makes no mention of the arrangement or number of atoms. It is standard for many ionic compounds, like calcium chloride (CaCl2), and for macromolecules, such as silicon dioxide (SiO2). The molecular formula, on the other hand, shows the number of each type of atom in a molecule. The structural formula shows the arrangement of the molecule. It is also possible for different types of compounds to have equal empirical formulas. In the early ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mixed-valence Compound
Mixed valence complexes contain an element which is present in more than one oxidation state. Well-known mixed valence compounds include the Creutz–Taube complex, Prussian blue, and molybdenum blue. Many solids are mixed-valency including indium chalcogenides. Robin–Day classification Mixed-valence compounds are subdivided into three groups, according to the Robin–Day classification: *Class I, where the valences are trapped—localized on a single site—such as Pb3O4 and antimony tetroxide. There are distinct sites with different specific valences in the complex that cannot easily interconvert. *Class II, which are intermediate in character. There is some localization of distinct valences, but there is a low activation energy for their interconversion. Some thermal activation is required to induce electron transfer from one site to another via the bridge. These species exhibit an intense Intervalence charge transfer (IT or IVCT) band, a broad intense absorption i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a base, and they are still among the most common bases. Etymology The word ''alkali'' is derived from Arabic ''al qalīy'' (or ''alkali''), meaning (see calcination), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly of potassium carbonate, was mildly basic. After heating this substance with calcium hydroxide (''slaked lime''), a far more strongly basic substance known as ''caustic potash ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Persulfate
Sodium persulfate is the inorganic compound with the formula Na2 S2 O8. It is the sodium salt of peroxydisulfuric acid, H2S2O8, an oxidizing agent. It is a white solid that dissolves in water. It is almost non-hygroscopic and has good shelf-life. Production The salt is prepared by the electrolytic oxidation of sodium bisulfate: : Oxidation is conducted at a platinum anode. In this way about 165,000 tons were produced in 2005. The standard redox potential of sodium persulfate into hydrogen sulfate is 2.1 V, which is higher than that of hydrogen peroxide (1.8 V) but lower than ozone (2.2 V). The sulfate radical formed in situ has a standard electrode potential of 2.7 V. However, there are a few drawbacks in utilizing platinum anodes to produce the salts; the manufacturing process is inefficient due to oxygen evolution and the product could contain contaminants coming from platinum corrosion (mainly due to extremely oxidizing nature of the sulfate radical). Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |