|
Potassium Nitrite
Potassium nitrite (distinct from potassium nitrate) is the inorganic compound with the chemical formula . It is an ionic salt of potassium ions K+ and nitrite ions NO2−, which forms a white or slightly yellow, hygroscopic crystalline powder that is soluble in water. It is a strong oxidizer and may accelerate the combustion of other materials. Like other nitrite salts such as sodium nitrite, potassium nitrite is toxic if swallowed, and laboratory tests suggest that it may be mutagenic or teratogenic. Gloves and safety glasses are usually used when handling potassium nitrite. Discovery Nitrite is present at trace levels in soil, natural waters, plant and animal tissues, and fertilizer. The pure form of nitrite was first made by the Swedish chemist Carl Wilhelm Scheele working in the laboratory of his pharmacy in the market town of Köping. He heated potassium nitrate at red heat for half an hour and obtained what he recognized as a new “salt.” The two compounds (potassium nitr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deliquescent
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Köping
''Köping'' was a Swedish denomination for a market town since the Middle Ages, derived from the Old Norse word ''kaupang''. The designation was officially abolished with the municipal reform of 1971, when Sweden was subdivided into the Municipalities of Sweden (currently amounting to 290). As present-day Finland was once a part of Sweden, the Finnish word ''kauppala'' has the same meaning. In modern Finnish, the word ''kaupunki'', borrowed from the Old Norse word ''kaupang'', is the main word for town and city. Swedish ''köping'' and the English toponym ''chipping'' are also cognates as is the Norwegian word ''kjøpstad'' and the Danish toponymical suffix '' -købing''. Sweden History In 1863 the first local government acts were implemented in Sweden. There were two acts, one for cities and one for rural areas. Of the around 2,500 municipalities, 89 had city rights and thus had the right to call themselves ''stad'' (city). Under the "rural" act there were also eight loca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Venesection
In medicine, venipuncture or venepuncture is the process of obtaining intravenous access for the purpose of venous blood sampling (also called ''phlebotomy'') or intravenous therapy. In healthcare, this procedure is performed by medical laboratory scientists, medical practitioners, some EMTs, paramedics, phlebotomists, dialysis technicians, and other nursing staff. In veterinary medicine, the procedure is performed by veterinarians and veterinary technicians. It is essential to follow a standard procedure for the collection of blood specimens to get accurate laboratory results. Any error in collecting the blood or filling the test tubes may lead to erroneous laboratory results. Venipuncture is one of the most routinely performed invasive procedures and is carried out for any of five reasons: # to obtain blood for diagnostic purposes; # to monitor levels of blood components; # to administer therapeutic treatments including medications, nutrition, or chemotherapy; # to remove ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Edinburgh Royal Infirmary
The Royal Infirmary of Edinburgh, or RIE, often (but incorrectly) known as the Edinburgh Royal Infirmary, or ERI, was established in 1729 and is the oldest voluntary hospital in Scotland. The new buildings of 1879 were claimed to be the largest voluntary hospital in the United Kingdom, and later on, the Empire."In Coming Days" The Edinburgh Royal Infirmary Souvenir Brochure 1942 The hospital moved to a new 900 bed site in 2003 in Little France. It is the site of clinical medicine teaching as well as a teaching hospital for the University of Edinburgh Medical School. In 1960, the first successful kidney transplant performed in the UK was at this hospital. In 1964, the world's first coronary care unit was established at the hospital. It is the only site for liver, pancreas and pancreatic islet cell transplantation and one of two sites for kidney transplantation in Scotland. In 2012, the Emergency Department had 113,000 patient attendances, the highest number in Scotland. It is man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angina Pectoris
Angina, also known as angina pectoris, is chest pain or pressure, usually caused by insufficient blood flow to the heart muscle (myocardium). It is most commonly a symptom of coronary artery disease. Angina is typically the result of obstruction or spasm of the arteries that supply blood to the heart muscle. The main mechanism of coronary artery obstruction is atherosclerosis as part of coronary artery disease. Other causes of angina include abnormal heart rhythms, heart failure and, less commonly, anemia. The term derives from the Latin ''angere'' ("to strangle") and ''pectus'' ("chest"), and can therefore be translated as "a strangling feeling in the chest". There is a weak relationship between severity of angina and degree of oxygen deprivation in the heart muscle, however, the severity of angina does not always match the degree of oxygen deprivation to the heart or the risk of a myocardial infarction (heart attack). Some people may experience severe pain even though the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobaltic Oxide
Cobalt(III) oxide is the inorganic compound with the formula of Co2O3. Although only two oxides of cobalt are well characterized, CoO and Co3O4, procedures claiming to give Co2O3 have been described. Thus treatment of Co(II) salts such as cobalt(II) sulfate with an aqueous solution of sodium hypochlorite (also known as bleach) gives a black solid: :2CoSO4 + 4NaOH + NaOCl → Co2O3 + 2Na2SO4 + NaCl Some formulations of the catalyst hopcalite contain "Co2O3". Some studies have been unable to synthesize the compound, and report that it is theoretically unstable. It is soluble in cold diluted sulfuric acid and produces Co2 O4sub>3, which is blue in aqueous solution. : Co2O3 + 3H2SO4 → Co2 O4sub>3 + 3H2O Colbalt(III) ion is a strong oxidizer in acidic solution, its standard electrode potential is +1.84V in this situation. See also *Cobalt Oxide Nanoparticles In materials and electric battery research, cobalt oxide nanoparticles usually refers to particles of cobalt( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferric Oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main source of iron for the steel industry. Fe2O3 is readily attacked by acids. Iron(III) oxide is often called rust, and to some extent this label is useful, because rust shares several properties and has a similar composition; however, in chemistry, rust is considered an ill-defined material, described as Hydrous ferric oxide. Structure Fe2O3 can be obtained in various polymorphs. In the main one, α, iron adopts octahedral coordination geometry. That is, each Fe center is bound to six oxygen ligands. In the γ polymorph, some of the Fe sit on tetrahedral sites, with four oxygen ligands. Alpha phase α-Fe2O3 has the rhombohedral, corundum (α-Al2O3) structure and i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Amide
Potassium amide is an inorganic compound with the chemical formula . Like other alkali metal amides, it is a white solid that hydrolyzes readily. It is a strong base. Production Potassium amide is produced by the reaction of ammonia with potassium. The reaction typically requires a catalyst. Structure Traditionally is viewed as a simple salt, but it has significant covalent character and is highly aggregated in ammonia solution. The compound has been characterized by X-ray crystallography X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ... as the solvent-free form as well as the mono- and diammonia solvates. In , the potassium centers are each bonded to two amido ligands and four ammonia ligands, all six of which bridge to adjacent potassium centers. The result is a chain of hexacoo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decomposition
Decomposition or rot is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is essential for recycling the finite matter that occupies physical space in the biosphere. Bodies of living organisms begin to decompose shortly after death. Animals, such as worms, also help decompose the organic materials. Organisms that do this are known as decomposers or detritivores. Although no two organisms decompose in the same way, they all undergo the same sequential stages of decomposition. The science which studies decomposition is generally referred to as ''taphonomy'' from the Greek word ''taphos'', meaning tomb. Decomposition can also be a gradual process for organisms that have extended periods of dormancy. One can differentiate abiotic decomposition from biotic decomposition (biodegradation). The former means "the degradation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. Historical introduction The concept of chemical equilibrium was developed in 1803, after Berthollet found that some chemical reactions are reversible. For any reaction mixture to exist at equilibrium, the rates of the forward and backward (reverse) reactions must be equal. In the following chemical equation, arrows point both ways to indicate equilibrium. A and B are reactant chemical species, S and T are p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
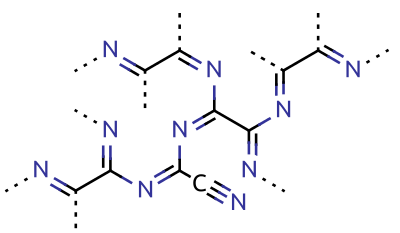
Cyanogen
Cyanogen is the chemical compound with the formula ( C N)2. It is a colorless and highly toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups – analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing. The two cyano groups are bonded together at their carbon atoms: N≡C‒ C≡N, although other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide (NCBr) (but see also ''Cyano radical''.) Cyanogen is the anhydride of oxamide: :H2NC(O)C(O)NH2 → NCCN + 2 H2O although oxamide is manufactured from cyanogen by hydrolysis: :NCCN + 2 H2O → H2NC(O)C(O)NH2 Preparation Cyanogen is typically generated from cyanide compounds. One laboratory method entails thermal decomposition of mercuric cyanide: :2 Hg(CN)2 → (CN)2 + Hg2(CN)2 Alternatively, one can combine solutions of copper(II) salts (such as copper(II) sulfate) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanamide
Cyanamide is an organic compound with the chemical formula, formula carbon, Cnitrogen, N2hydrogen, H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol-deterrent drug. The molecule features a nitrile group attached to an amino group. Derivatives of this compound are also referred to as cyanamides, the most common being calcium cyanamide (CaCN2). Tautomers and self-condensations Containing both a nucleophilic and electrophilic site within the same molecule, cyanamide undergoes various reactions with itself. Cyanamide exists as two tautomers, one with the connectivity N≡C–NH2 and the other with the formula HN=C=NH ("carbodiimide" tautomer). The N≡C–NH2 form dominates, but in a few reactions (e.g. silylation) the diimide form appears to be important. Cyanamide dimerizes to give 2-Cyanoguanidine, 2-cyanoguanidine (dicyandiamide). This dimerization is disfavored by acids and is inhi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |