|
Phenylethanolamine Ethers
Phenylethanolamine (sometimes abbreviated PEOH), or β-hydroxyphenethylamine, is a trace amine with a structure similar to those of other trace phenethylamines as well as the catecholamine neurotransmitters dopamine, norepinephrine, and epinephrine. As an organic compound, phenylethanolamine is a β-hydroxylated phenethylamine that is also structurally related to a number of synthetic drugs in the substituted phenethylamine class. In common with these compounds, phenylethanolamine has strong cardiovascular activity and, under the name ''Apophedrin'', has been used as a drug to produce topical vasoconstriction.''The Merck Index, 10th Ed.'' (1983), p. 1051, Merck & Co., Rahway. In appearance, phenylethanolamine is a white solid. Phenylethanolamine is perhaps best known in the field of bioscience as part of the enzyme name " phenylethanolamine N-methyl transferase", referring to an enzyme which is responsible for the conversion of norepinephrine into epinephrine, as well as other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Amine
Trace amines are an endogenous group of trace amine-associated receptor 1 (TAAR1) agonists – and hence, monoaminergic neuromodulators – that are structurally and metabolically related to classical monoamine neurotransmitters. Compared to the classical monoamines, they are present in trace concentrations. They are distributed heterogeneously throughout the mammalian brain and peripheral nervous tissues and exhibit high rates of metabolism. Although they can be synthesized within parent monoamine neurotransmitter systems, there is evidence that suggests that some of them may comprise their own independent neurotransmitter systems. Trace amines play significant roles in regulating the quantity of monoamine neurotransmitters in the synaptic cleft of monoamine neurons with . They have well-characterized presynaptic ''amphetamine-like'' effects on these monoamine neurons via TAAR1 activation; specifically, by activating TAAR1 in neurons they promote the release and prevent reuptak ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Weak Base
A weak base is a base that, upon dissolution in water, does not dissociate completely, so that the resulting aqueous solution contains only a small proportion of hydroxide ions and the concerned basic radical, and a large proportion of undissociated molecules of the base. pH, Kb, and Kw Bases yield solutions in which the hydrogen ion activity is lower than it is in pure water, i.e., the solution is said to have a pH greater than 7.0 at standard conditions, potentially as high as 14 (and even greater than 14 for some bases). The formula for pH is: :\mbox = -\log_ \left \mbox^+ \right/math> Bases are proton acceptors; a base will receive a hydrogen ion from water, H2O, and the remaining H+ concentration in the solution determines pH. A weak base will have a higher H+ concentration than a stronger base because it is less completely protonated than a stronger base and, therefore, more hydrogen ions remain in its solution. Given its greater H+ concentration, the formula yields a lower ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
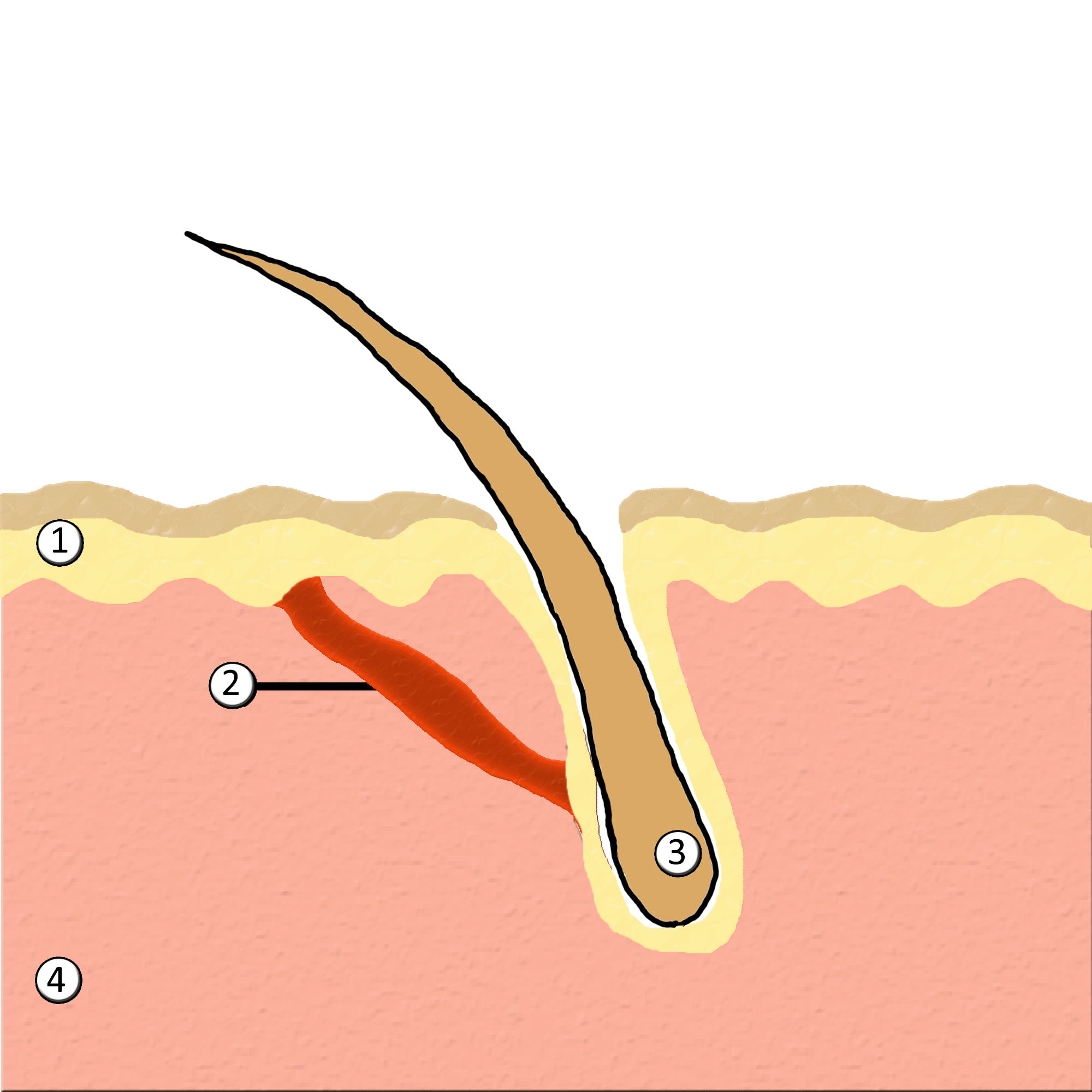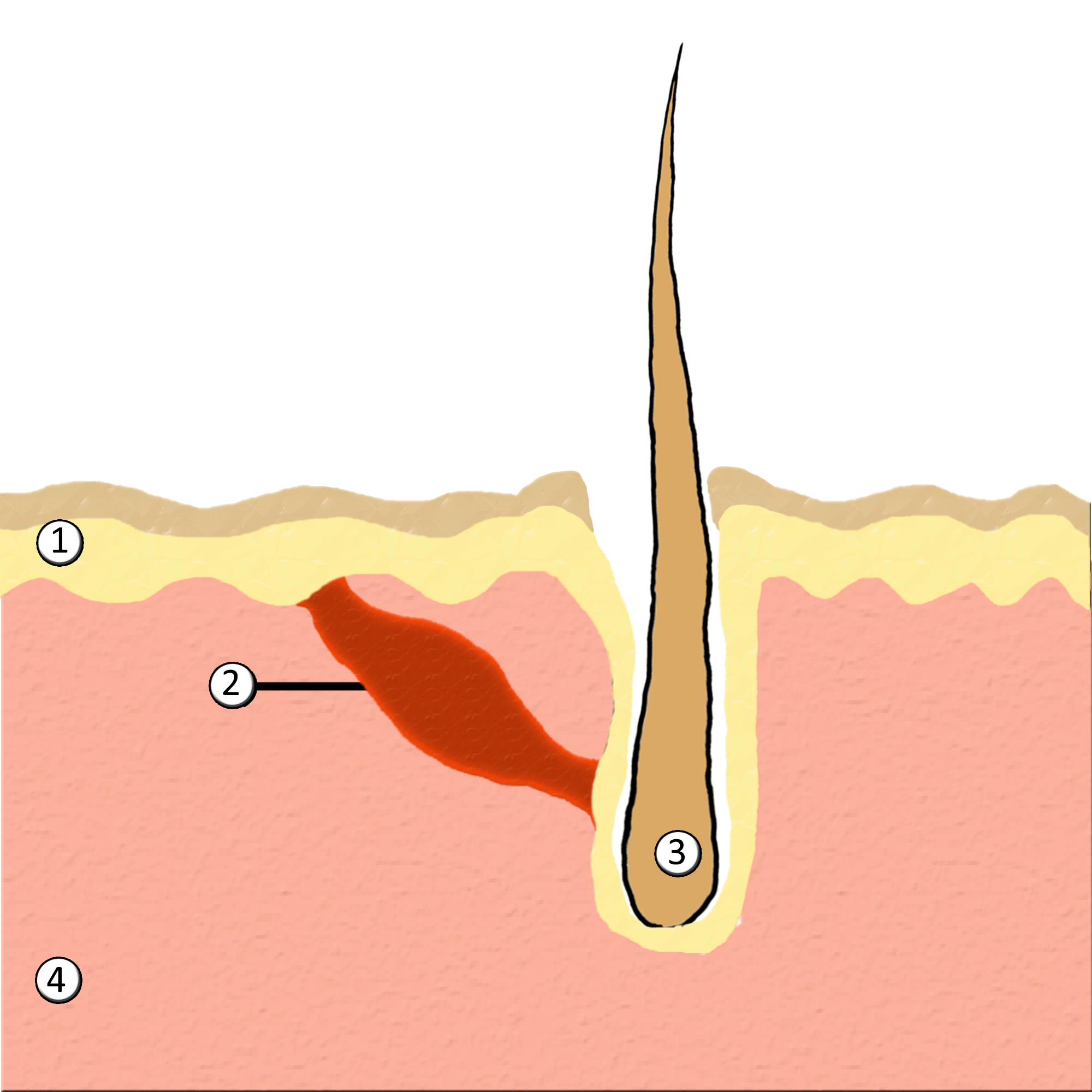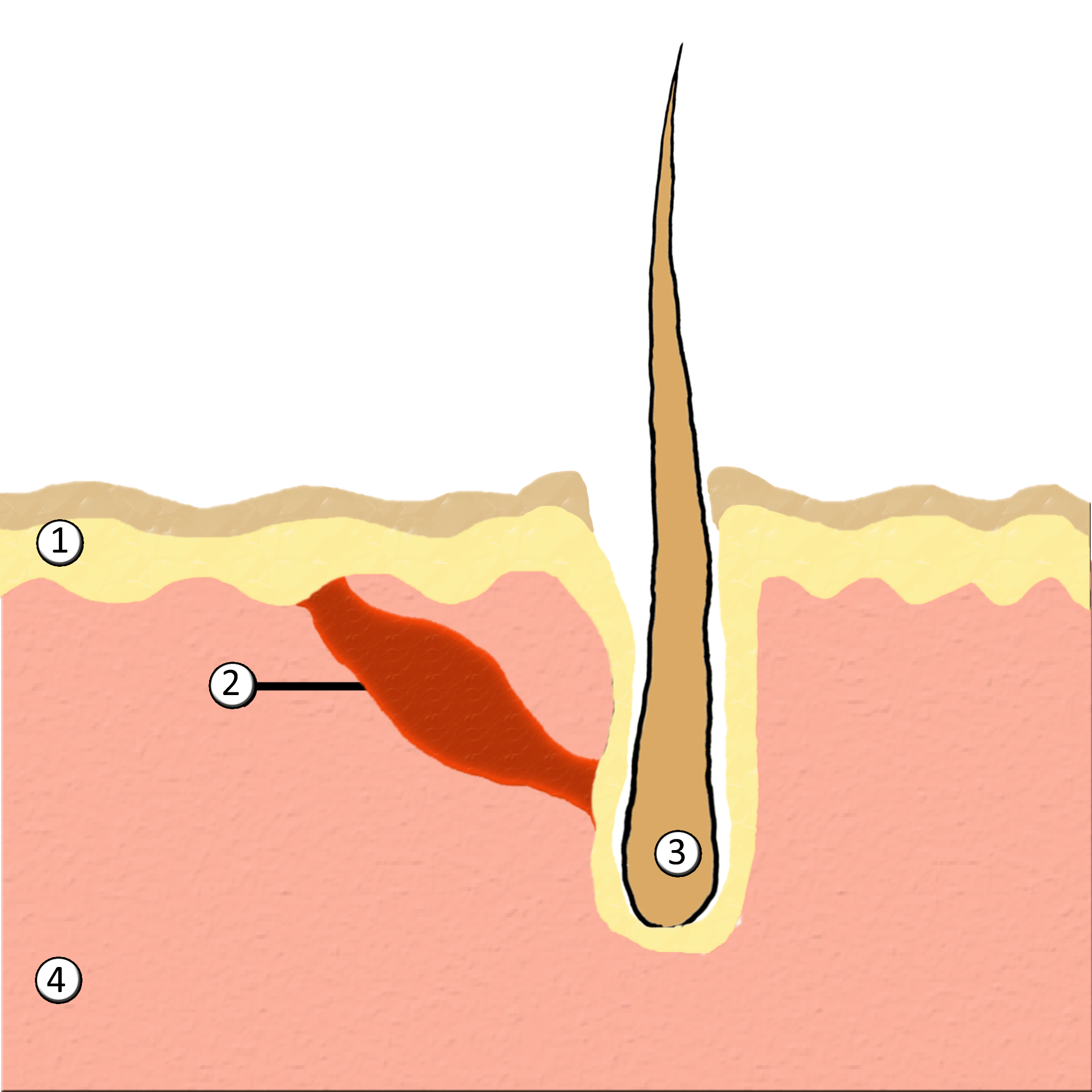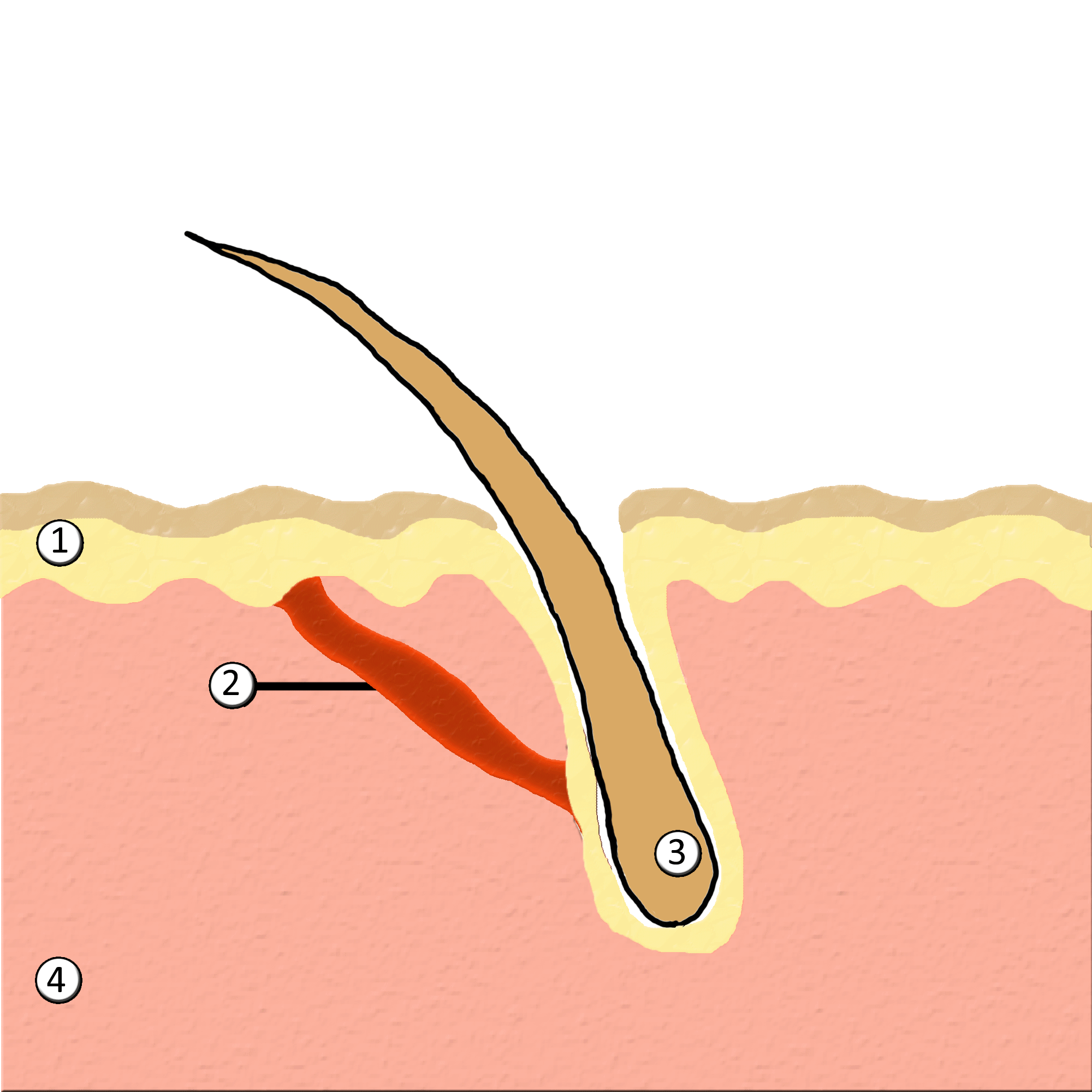
Piloerection
Goose bumps, goosebumps or goose-pimples are the bumps on a person's skin at the base of body hairs which may involuntarily develop when a person is tickled, cold or experiencing strong emotions such as fear, euphoria or sexual arousal. The formation of goose bumps in humans under stress is considered to be a vestigial reflex. Its function in other apes is to raise the body's hair, and would have made human ancestors appear larger to scare off predators or to increase the amount of air trapped in the fur to make it more insulating. The reflex of producing goose bumps is known as piloerection or the pilomotor reflex, or, more traditionally, horripilation. It occurs in many mammals; a prominent example is porcupines Porcupines are large rodents with coats of sharp spines, or quills, that protect them against predation. The term covers two families of animals: the Old World porcupines of family Hystricidae, and the New World porcupines of family, Erethiz ..., which raise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mydriatic
Mydriasis is the dilation of the pupil, usually having a non-physiological cause, or sometimes a physiological pupillary response. Non-physiological causes of mydriasis include disease, trauma, or the use of certain types of drugs. Normally, as part of the pupillary light reflex, the pupil dilates in the dark and constricts in the light to respectively improve vividity at night and to protect the retina from sunlight damage during the day. A ''mydriatic'' pupil will remain excessively large even in a bright environment. The excitation of the radial fibres of the iris which increases the pupillary aperture is referred to as a mydriasis. More generally, mydriasis also refers to the natural dilation of pupils, for instance in low light conditions or under sympathetic stimulation. Fixed, unilateral mydriasis could be a symptom of raised intracranial pressure. The opposite, constriction of the pupil, is referred to as miosis. Both mydriasis and miosis can be physiological. Anisocoria ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bronchodilator
A bronchodilator or broncholytic (although the latter occasionally includes secretory inhibition as well) is a substance that dilates the bronchi and bronchioles, decreasing resistance in the respiratory airway and increasing airflow to the lungs. Bronchodilators may be originating naturally within the body, or they may be medications administered for the treatment of breathing difficulties, usually in the form of inhalers. They are most useful in obstructive lung diseases, of which asthma and chronic obstructive pulmonary disease are the most common conditions. Although this remains somewhat controversial, they might be useful in bronchiolitis and bronchiectasis. They are often prescribed but of unproven significance in restrictive lung diseases. Bronchodilators are either short-acting or long-acting. Short-acting medications provide quick or "rescue" relief from acute bronchoconstriction. Long-acting bronchodilators help to control and prevent symptoms. The three types of pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Subcutaneous Injection
Subcutaneous administration is the insertion of medications beneath the skin either by injection or infusion. A subcutaneous injection is administered as a bolus into the subcutis, the layer of skin directly below the dermis and epidermis, collectively referred to as the cutis. The instruments are usually a hypodermic needle and a syringe. Subcutaneous injections are highly effective in administering medications such as insulin, morphine, diacetylmorphine and goserelin. Subcutaneous administration may be abbreviated as SC, SQ, subcu, sub-Q, SubQ, or subcut. Subcut is the preferred abbreviation to reduce the risk of misunderstanding and potential errors. Subcutaneous tissue has few blood vessels and so drugs injected here are for slow, sustained rates of absorption, often with some amount of depot effect. Compared with other routes of administration, it is slower than intramuscular injections but still faster than intradermal injections. Subcutaneous infusion (as opposed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mandelic Acid
Mandelic acid is an aromatic alpha hydroxy acid with the molecular formula C6H5CH(OH)CO2H. It is a white crystalline solid that is soluble in water and polar organic solvents. It is a useful precursor to various drugs. The molecule is chiral. The racemic mixture is known as ''paramandelic acid''. Isolation, synthesis, occurrence Mandelic acid was discovered in 1831 by the German pharmacist Ferdinand Ludwig Winckler (1801–1868) while heating amygdalin, an extract of bitter almonds, with diluted hydrochloric acid. The name is derived from the German "Mandel" for "almond". Mandelic acid is usually prepared by the acid-catalysed hydrolysis of mandelonitrile, which is the cyanohydrin of benzaldehyde. Mandelonitrile can also be prepared by reacting benzaldehyde with sodium bisulfite to give the corresponding adduct, forming mandelonitrile with sodium cyanide, which is hydrolyzed: : Alternatively, it can be prepared by base hydrolysis of phenylchloroacetic acid as well as dibromac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Racemate
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. History The first known racemic mixture was racemic acid, which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid. He manually separated the crystals of a mixture by hand, starting from an aqueous solution of the sodium ammonium salt of racemate tartaric acid. Pasteur benefited from the fact that ammonium tartrate salt that gives enantiomeric crystals with distinct crystal forms (at 77 °F). Reasoning from the macroscopic scale down to the molecular, he reckoned that the molecules had to have non-superimposable mirror images. A sample with only a single enantiomer is an ''enantiomerically pure'' or ''enantiopure'' compound. Etymology From racemic acid found in grapes; from Latin ''racemus'', meani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cahn–Ingold–Prelog Priority Rules
In organic chemistry, the Cahn–Ingold–Prelog (CIP) sequence rules (also the CIP priority convention; named for R.S. Cahn, C.K. Ingold, and Vladimir Prelog) are a standard process to completely and unequivocally name a stereoisomer of a molecule. The purpose of the CIP system is to assign an ''R'' or ''S'' descriptor to each stereocenter and an ''E'' or ''Z'' descriptor to each double bond so that the configuration of the entire molecule can be specified uniquely by including the descriptors in its systematic name. A molecule may contain any number of stereocenters and any number of double bonds, and each usually gives rise to two possible isomers. A molecule with an integer describing the number of stereocenters will usually have stereoisomers, and diastereomers each having an associated pair of enantiomers. The CIP sequence rules contribute to the precise naming of every stereoisomer of every organic molecule with all atoms of ligancy of fewer than 4 (but includi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dextrorotatory
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circular birefringence and circular dichroism are the manifestations of optical activity. Optical activity occurs only in chiral materials, those lacking microscopic mirror symmetry. Unlike other sources of birefringence which alter a beam's state of polarization, optical activity can be observed in fluids. This can include gases or solutions of chiral molecules such as sugars, molecules with helical secondary structure such as some proteins, and also chiral liquid crystals. It can also be observed in chiral solids such as certain crystals with a rotation between adjacent crystal planes (such as quartz) or metamaterials. When looking at the source of light, the rotation of the plane of polarization may be either to the right (dextrorotatory ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Racemic Mixture
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. History The first known racemic mixture was racemic acid, which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid. He manually separated the crystals of a mixture by hand, starting from an aqueous solution of the sodium ammonium salt of racemate tartaric acid. Pasteur benefited from the fact that ammonium tartrate salt that gives enantiomeric crystals with distinct crystal forms (at 77 °F). Reasoning from the macroscopic scale down to the molecular, he reckoned that the molecules had to have non-superimposable mirror images. A sample with only a single enantiomer is an ''enantiomerically pure'' or ''enantiopure'' compound. Etymology From racemic acid found in grapes; from Latin ''racemus'', meani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |