|
Photographic Fixer
Photographic fixer is a mixture of chemicals used in the final step in the photographic processing of film or paper. The fixer stabilises the image, removing the unexposed silver halide remaining on the photographic film or photographic paper, leaving behind the reduced metallic silver that forms the image. By fixation, the film or paper is insensitive to further action by light. Without fixing, the remaining silver halide would darken and cause fogging of the image. Chemistry Fixation is commonly achieved by treating the film or paper with a solution of thiosulfate salt. Popular salts are sodium thiosulfate—commonly called hypo—and ammonium thiosulfate—commonly used in modern rapid fixer formulae. Fixation by thiosulfate involves these chemical reactions (X = halide, typically Br−):Karlheinz Keller et al. "Photography" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. . :AgX + 2 S2O32− → g(S2O3)2sup>3− + X− :AgX + 3 S2O32− → ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Self Portrait In The Darkroom JPG01
In philosophy, the self is an individual's own being, knowledge, and values, and the relationship between these attributes. The first-person perspective distinguishes selfhood from personal identity. Whereas "identity" is (literally) sameness and may involve categorization and labeling, selfhood implies a first-person perspective and suggests potential uniqueness. Conversely, "person" is used as a third-person reference. Personal identity can be impaired in late-stage Alzheimer's disease and in other neurodegenerative diseases. Finally, the self is distinguishable from "others". Including the distinction between sameness and otherness, the self versus other is a research topic in contemporary philosophy and contemporary phenomenology (see also psychological phenomenology), psychology, psychiatry, neurology, and neuroscience. Although subjective experience is central to selfhood, the privacy of this experience is only one of many problems in the philosophy of self and sci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
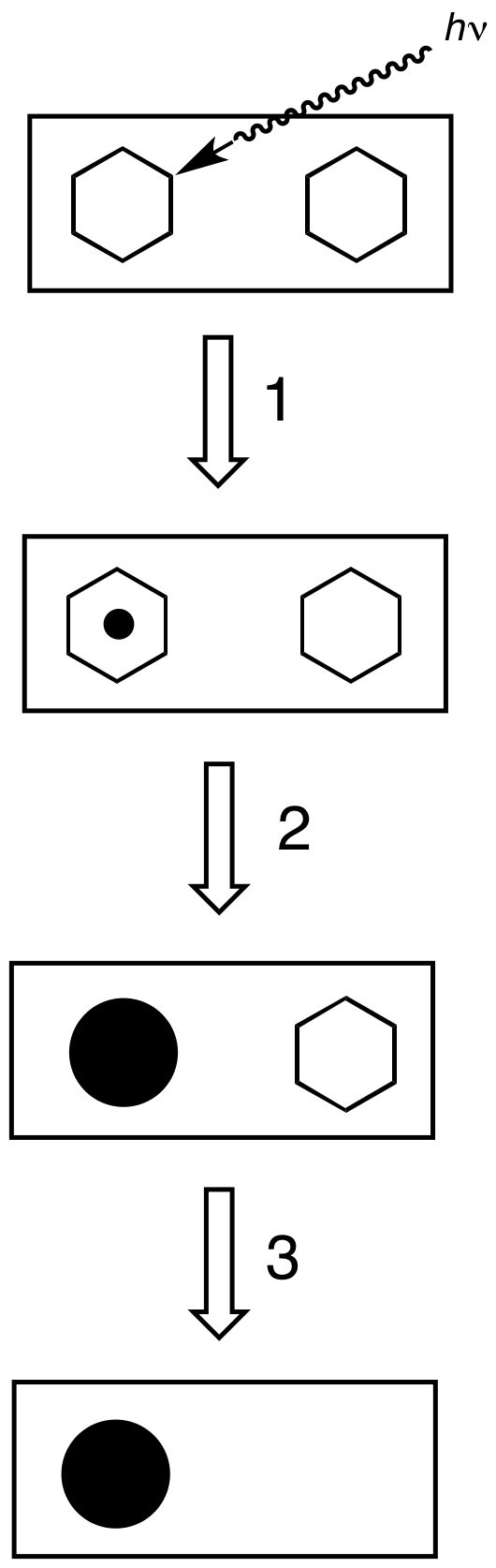
Photographic Developer
In the Photographic processing, processing of photographic films, plates or papers, the photographic developer (or just developer) is one or more chemicals that convert the latent image to a visible image. Developing agents achieve this conversion by Redox, reducing the silver halides, which are pale-colored, into silver metal, which is black when in the form of fine particles.Karlheinz Keller et al. ''Photography'' in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2005, Wiley-VCH, Weinheim. . The conversion occurs within the gelatine matrix. The special feature of photography is that the developer acts more quickly on those particles of silver halide that have been exposed to light. When left in developer, all the silver halides will eventually be reduced and turn black. Generally, the longer a developer is allowed to work, the darker the image. Chemical composition of developers The developer typically consists of a mixture of chemical compounds prepared as an aqueous solut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Washing (photography)
In photography, washing is an important part of all film processing and printmaking processes. After materials have been fixed, washing removes unwanted and exhausted processing chemicals which, if left in situ, may cause deterioration and destruction of the image. A disadvantage of the use of thiosulfate as a fixer is its ability to dissolve elemental silver at a very slow rate. If films or papers are inadequately washed after fixing, any residual fixer can slowly bleach or stain the photographic image. For prints on high grade fibre papers, a period of continuous washing in clean, cold water for up to 40 minutes may be required. For modern plastic (resin) coated papers, washing for as little as 2 minutes in warm water can be sufficient to eliminate residual fixer. Washing aids (also called hypo clearing agents) can be used to make the process of removing fixer faster and more thorough. A quick, water-saving, and archival technique for washing film fixed ''with nonhardening fixer'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelate
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity. The word ''chelation'' is derived from Greek χηλή, ''chēlē'', meaning "claw"; the ligands lie around the central atom like the claws of a crab. The term ''chelate'' () was first applied in 1920 by Sir Gilbert T. Morgan and H. D. K. Drew, who stated: "The adjective chelate, derived from the great claw or ''chele'' (Greek) of the crab or other crustaceans, is suggested for the caliperlike groups which function as two associating units and fasten to the central atom so as to produce heterocyclic rings." Chelation is useful in applications such as providing nutritional supplements, in chela ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EDTA
Ethylenediaminetetraacetic acid (EDTA), also called EDTA acid, is an aminopolycarboxylic acid with the formula . This white, slightly water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes even at neutral pH. It is thus used to dissolve Fe- and Ca-containing scale as well as to deliver iron ions under conditions where its oxides are insoluble. EDTA is available as several salts, notably disodium EDTA, sodium calcium edetate, and tetrasodium EDTA, but these all function similarly. Uses EDTA is widely used in industry. It also has applications in food preservation, medicine, cosmetics, water softening, in laboratories, and other fields. Industrial EDTA is mainly used to sequester (bind or confine) metal ions in aqueous solution. In the textile industry, it prevents metal ion impurities from modifying colours of dyed products. In the pulp and paper industry, EDTA inhibits the ability of metal ions, especiall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromogenic
In chemistry, the term chromogen refers to a colourless (or faintly coloured) chemical compound that can be converted by chemical reaction into a compound which can be described as "coloured" (a chromophore). There is no universally agreed definition of the term. Various dictionaries give the following definitions: * A substance capable of conversion into a pigment or dye. * Any substance that can become a pigment or coloring matter, a substance in organic fluids that forms colored compounds when oxidized, or a compound, not itself a dye, that can become a dye. * Any substance, itself without color, giving origin to a coloring matter. In biochemistry the term has a rather different meaning. The following are found in various dictionaries. * A precursor of a biochemical pigment * A pigment-producing microorganism * Any of certain bacteria that produce a pigment * A strongly pigmented or pigment-generating organelle, organ, or microorganism. Applications in chemistry *In chromogenic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kodachrome
Kodachrome is the brand name for a color reversal film introduced by Eastman Kodak in 1935. It was one of the first successful color materials and was used for both cinematography and still photography. For many years, Kodachrome was widely used for professional color photography, especially for images intended for publication in print media. Because of its complex processing requirements, the film was initially sold only with the cost of processing; independent photography stores were prohibited from developing Kodachrome. To develop the film, customers had to mail it to Kodak, which would then send the developed film back as part of the purchase price. In 1954, the U.S. Department of Justice found that this practice violated antitrust laws by being uncompetitive. Kodak then entered into a consent decree, requiring the company to offer Kodachrome film for sale without the development fee, as well as license Kodachrome development patents to independent photography stores. Kodak ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bisulfite
The bisulfite ion (IUPAC-recommended nomenclature: hydrogensulfite) is the ion . Salts containing the ion are also known as "sulfite lyes". Sodium bisulfite is used interchangeably with sodium metabisulfite (Na2S2O5). Sodium metabisulfite dissolves in water to give a solution of Na+. :Na2S2O5 + H2O → 2Na SO3 Structure The bisulfite anion exists in solution as a mixture of two tautomers. One tautomer has the proton attached to one of the three oxygen atoms. In the second tautomer the proton resides on sulfur. The S-protonated tautomer has ''C''3v symmetry. The O-protonated tautomer has only ''C''s symmetry. Reactions Tautomerization There exist two tautomers of bisulfite. They interconvert readily but can be characterized individually by various spectroscopic methods. They have been observed by 17O NMR spectroscopy: :HSO3− SO2(OH)− ''K'' = 4.9 Acid-base reactions Solutions of bisulfite are typically prepared by treatment of sulfur dioxide with aqueous bas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluoride, chloride, bromide, iodide, astatide, or theoretically tennesside compound. The alkali metals combine directly with halogens under appropriate conditions forming halides of the general formula, MX (X = F, Cl, Br or I). Many salts are halides; the ''hal-'' syllable in ''halide'' and '' halite'' reflects this correlation. A halide ion is a halogen atom bearing a negative charge. The common halide anions are fluoride (), chloride (), bromide (), and iodide (). Such ions are present in many ionic halide salts. Halide minerals contain halides. All these halide anions are colorless. Halides also form covalent bonds, examples being colorless TiF4, colorless TiCl4, orange TiBr4, and brown TiI4. The heavier members TiCl4, TiBr4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photographic Processing
Photographic processing or photographic development is the chemical means by which photographic film or paper is treated after photographic exposure to produce a negative or positive image. Photographic processing transforms the latent image into a visible image, makes this permanent and renders it insensitive to light.Karlheinz Keller et al. "Photography" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. All processes based upon the gelatin silver process are similar, regardless of the film or paper's manufacturer. Exceptional variations include instant films such as those made by Polaroid and thermally developed films. Kodachrome required Kodak's proprietary K-14 process. Kodachrome film production ceased in 2009, and K-14 processing is no longer available as of December 30, 2010. Ilfochrome materials use the dye destruction process. Deliberately using the wrong process for a film is known as cross processing. Common processes All p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Thiosulfate
Ammonium thiosulfate (ammonium thiosulphate in British English) is an inorganic compound with the formula . It is white crystalline solid with ammonia odor, readily soluble in water, slightly soluble in acetone and insoluble in ethanol and diethyl ether. Production It is produced by treating ammonium sulfite with sulfur at temperatures between 85 and 110 °C: : Applications Ammonium thiosulfate is used in photographic fixer. It is a so-called rapid fixer, acting more quickly than sodium thiosulfate fixers. Fixation involves these chemical reactions (illustrated for silver bromide): : : Also exploiting the stability of thiosulfate coordination complexes, ammonium thiosulfate is also used for leaching of gold and silver. It works with presence of copper as a catalyst. This process is a nontoxic alternative gold cyanidation. The advantage to ammonium thiosulfate is that the pyrolysis of its silver complexes leaves a residue solely of silver sulfide, in contrast to complexes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Thiosulfate
Sodium thiosulfate (sodium thiosulphate) is an inorganic compound with the formula . Typically it is available as the white or colorless pentahydrate (x = 5), which is a white solid that dissolves well in water. The compound is a reducing agent and a ligand, and these properties underpin its applications. Uses Sodium thiosulfate is used predominantly in dyeing. It converts some dyes to their soluble colorless "leuco" forms. It is also used to bleach "wool, cotton, silk, ...soaps, glues, clay, sand, bauxite, and... edible oils, edible fats, and gelatin." Medical uses Sodium thiosulfate is used in the treatment of cyanide poisoning. It is on the World Health Organization's List of Essential Medicines. Other uses include topical treatment of ringworm and tinea versicolor, and treating some side effects of hemodialysis and chemotherapy. In September 2022, the U.S. Food and Drug Administration (FDA) approved sodium thiosulfate under the trade name Pedmark to lessen the risk of oto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |