|
Photochromism
Photochromism is the reversible change of color upon exposure to light. It is a transformation of a chemical species (photoswitch) between two forms through the absorption of electromagnetic radiation (photoisomerization), where each form has a different absorption spectrum. This reversible structural or geometric change in photochromic molecules affects their electronic configuration, molecular strain energy, and other properties. History In 1867, Carl Julius Fritzsche reported the concept of photochromism, indicating that orange tetracene solution lost its color in daylight but regained it in darkness. Later, similar behavior was observed by both Edmund ter Meer and Phipson. Ter Meer documented the color change of the potassium salt of dinitroethane, which appeared red in daylight and yellow in the dark. Phipson also recorded that a painted gatepost appeared black during the day and white at night due to a zinc pigment, likely lithopone. In 1899, Willy Markwald, who studied th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spiropyran
A spiropyran is a type of photochromic organic chemical compound, characterized by their ability to reversibly switch between two structural forms—spiropyran and merocyanine—upon exposure to light or other external stimuli. This reversible transformation alters their optical and electronic properties, making them valuable in various applications, including molecular switches, optical data storage, sensors, and smart materials. History Spiropyrans were discovered in the early twentieth century, but it was not until 1952 that their photochromic properties were formally documented by chemists Fischer and Gerhard Hirshberg. Their pioneering work demonstrated that spiropyrans undergo reversible structural and color changes when exposed to ultraviolet light, a phenomenon that sparked widespread interest in photoresponsive organic compounds. Throughout the latter half of the twentieth century, advancements in synthetic methods enabled the development of a wide range of spiropyran deriv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photochromic Dye Transition
Photochromism is the reversible change of color upon exposure to light. It is a transformation of a chemical species (photoswitch) between two forms through the absorption of electromagnetic radiation (photoisomerization), where each form has a different absorption spectrum. This reversible structural or geometric change in photochromic molecules affects their electronic configuration, molecular strain energy, and other properties. History In 1867, Carl Julius Fritzsche reported the concept of photochromism, indicating that orange tetracene solution lost its color in daylight but regained it in darkness. Later, similar behavior was observed by both Edmund ter Meer and Phipson. Ter Meer documented the color change of the potassium salt of dinitroethane, which appeared red in daylight and yellow in the dark. Phipson also recorded that a painted gatepost appeared black during the day and white at night due to a zinc pigment, likely lithopone. In 1899, Willy Markwald, who studied th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3D Optical Data Storage
3D optical data storage is any form of optical data storage in which information can be recorded or read with three-dimensional resolution (as opposed to the two-dimensional resolution afforded, for example, by CD). This innovation has the potential to provide petabyte-level mass storage on DVD-sized discs (120mm). Data recording and readback are achieved by focusing lasers within the medium. However, because of the volumetric nature of the data structure, the laser light must travel through other data points before it reaches the point where reading or recording is desired. Therefore, some kind of nonlinearity is required to ensure that these other data points do not interfere with the addressing of the desired point. No commercial product based on 3Doptical data storage has yet arrived on the mass market, although several companies are actively developing the technology and claim that it may become available 'soon'. Overview Current optical data storage media, such as the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithienylethene
Diarylethene is the general name of a class of chemical compounds that have aromatic functional groups bonded to each end of a carbon–carbon double bond. The simplest example is stilbene, which has two geometric isomers, E and Z. Under the influence of light, these compounds can generally perform two kinds of reversible isomerizations: * E to Z isomerizations, most common for stilbenes (and azobenzenes). This process goes through an excited state energy minimum where the aromatic rings lie at 90° to each other. This conformation drops to the ground state and generally relaxes to trans and cis forms in a 1:1 ratio, thus the quantum yield for E-Z isomerization is very rarely greater than 0.5. *6π electrocyclizations of the Z form, leading to an additional bond between the two aryl functionalities and a disruption of the aromatic character of these groups.J. March, ''Advanced Organic Chemistry'', 4th ed. (1992). The quantum yield of this reaction is generally less than 0.1, and in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photostationary State
The photostationary state of a reversible photochemical reaction is the equilibrium chemical composition under a specific kind of electromagnetic irradiation (usually a single wavelength of visible or UV radiation). It is a property of particular importance in photochromic compounds, often used as a measure of their practical efficiency and usually quoted as a ratio or percentage. The position of the photostationary state is primarily a function of the irradiation parameters, the absorbance spectra of the chemical species, and the quantum yields of the reactions. The photostationary state can be very different from the composition of a mixture at thermodynamic equilibrium. As a consequence, photochemistry can be used to produce compositions that are "contra-thermodynamic". For instance, although ''cis''-stilbene is "uphill" from ''trans-''stilbene in a thermodynamic sense, irradiation of ''trans''-stilbene results in a mixture that is predominantly the ''cis'' isomer. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Transfer
Electron transfer (ET) occurs when an electron relocates from an atom, ion, or molecule, to another such chemical entity. ET describes the mechanism by which electrons are transferred in redox reactions. Electrochemical processes are ET reactions. ET reactions are relevant to photosynthesis and respiration and commonly involve transition metal complexes. In organic chemistry ET is a step in some industrial polymerization reactions. It is foundational to photoredox catalysis. Classes of electron transfer Inner-sphere electron transfer In inner-sphere ET, two redox centers are covalently linked during the ET. This bridge can be permanent, in which case the electron transfer event is termed intramolecular electron transfer. More commonly, however, the covalent linkage is transitory, forming just prior to the ET and then disconnecting following the ET event. In such cases, the electron transfer is termed intermolecular electron transfer. A famous example of an inner sphere ET pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Dipole Moment
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system: that is, a measure of the system's overall Chemical polarity, polarity. The International System of Units, SI unit for electric dipole moment is the coulomb-metre (C⋅m). The debye (D) is another unit of measurement used in atomic physics and chemistry. Theoretically, an electric dipole is defined by the first-order term of the multipole expansion; it consists of two equal and opposite charges that are infinitesimally close together, although real dipoles have separated charge.Many theorists predict elementary particles can have very tiny electric dipole moments, possibly without separated charge. Such small dipoles make no difference to everyday physics, and have not yet been observed (see ''Electron electric dipole moment''). However, when making measurements at a distance much larger than the charge separation, the dipole gives a good approximation of the actua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
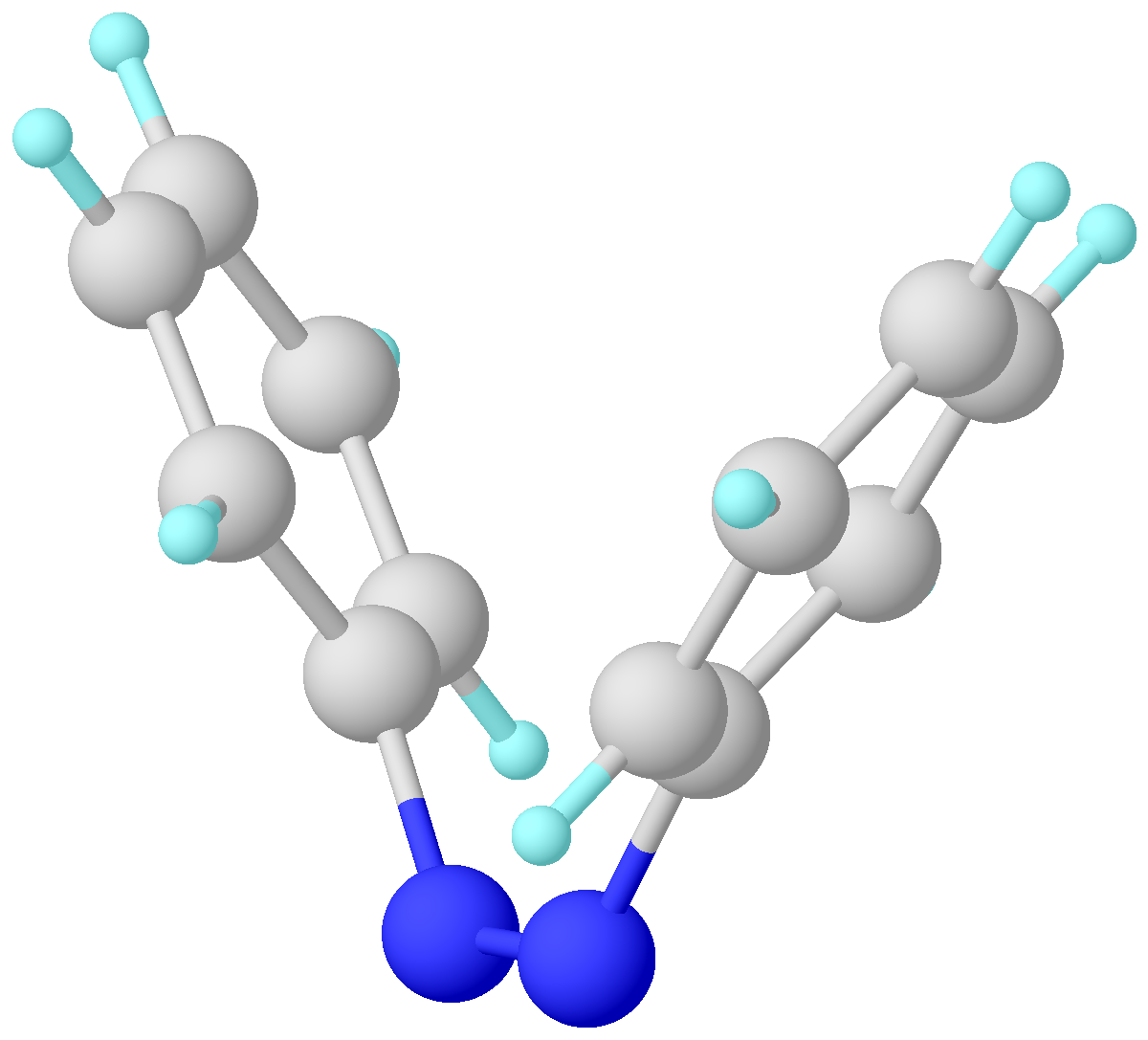
Azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a azo compound, N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar Chemical compound, compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Different classes of azo dyes exist, most notably the ones substituted with heteroaryl rings. Structure and synthesis Azobenzene was first described by Eilhard Mitscherlich in 1834. Yellowish-red crystalline flakes of azobenzene were obtained in 1856. Its original preparation is similar to the modern one. According to the 1856 method, nitrobenzene is reduced by iron filings in the presence of acetic acid. In the modern synthesis, zinc is the reductant in the presence of a base. Industrial electrosynthesis using nitrobenzene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photobleaching
In optics, photobleaching (sometimes termed fading) is the photochemical alteration of a dye or a fluorophore molecule such that it is permanently unable to fluoresce. This is caused by cleaving of covalent bonds or non-specific reactions between the fluorophore and surrounding molecules. Such irreversible modifications in covalent bonds are caused by transition from a singlet state to the triplet state of the fluorophores. The number of excitation cycles to achieve full bleaching varies. In microscopy, photobleaching may complicate the observation of fluorescent molecules, since they will eventually be destroyed by the light exposure necessary to stimulate them into fluorescing. This is especially problematic in time-lapse microscopy. However, photobleaching may also be used prior to applying the (primarily antibody-linked) fluorescent molecules, in an attempt to quench autofluorescence. This can help improve the signal-to-noise ratio. Photobleaching may also be exploited to s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |