|
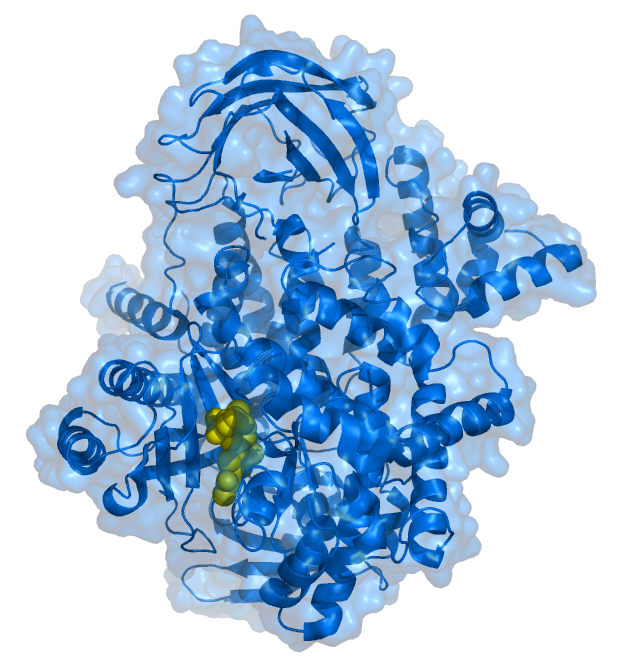
Phosphoinositide 3-kinase Inhibitor
Phosphoinositide 3-kinase inhibitors (PI3K inhibitors) are a class of medical drugs that are mainly used to treat advanced cancers. They function by inhibiting one or more of the phosphoinositide 3-kinase (PI3K) enzymes, which are part of the PI3K/AKT/mTOR pathway. This signal pathway regulates cellular functions such as growth and survival. It is strictly regulated in healthy cells, but is always active in many cancer cells, allowing the cancer cells to better survive and multiply. PI3K inhibitors block the PI3K/AKT/mTOR pathway and thus slow down cancer growth. They are examples of a targeted therapy. While PI3K inhibitors are an effective treatment, they can have very severe side effects and are therefore only used if other treatments have failed or are not suitable. After PI3K inhibitors had been under investigation as anti-cancer drugs for several years, the first one to be approved for treatment in clinical practice was idelalisib in 2014. Several others followed, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PI3K Inhibitors Overview Mishra2021
Phosphoinositide 3-kinases (PI3Ks), also called phosphatidylinositol 3-kinases, are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, survival and intracellular trafficking, which in turn are involved in cancer. PI3Ks are a family of related intracellular signal transducer enzymes capable of phosphorylating the 3 position hydroxyl group of the inositol ring of phosphatidylinositol (PtdIns). The pathway, with oncogene PIK3CA and Tumor suppressor, tumor suppressor gene PTEN (gene), PTEN, is implicated in the sensitivity of cancer tumors to insulin and IGF1, and in calorie restriction. Discovery The discovery of PI3Ks by Lewis C. Cantley, Lewis Cantley and colleagues began with their identification of a previously unknown phosphoinositide kinase associated with the polyoma middle T protein. They observed unique substrate specificity and chromatographic properties of the products of the lipid kinase, leading to the disc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C). However, the agency also enforces other laws, notably Section 361 of the Public Health Service Act as well as associated regulations. Much of this regulatory-enforcement work is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase III Clinical Trial
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Description Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. When expressed specifically, a clinical trial phase is capitalized both in name and Roman numeral, such as "Phase I" clinical trial. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory au ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PASLI Disease
PASLI disease is a rare genetic disorder of the immune system. PASLI stands for “p110 delta activating mutation causing senescent T cells, lymphadenopathy, and immunodeficiency.” The immunodeficiency manifests as recurrent infections usually starting in childhood. These include bacterial infections of the respiratory system and chronic viremia due to Epstein–Barr virus (EBV) and/or cytomegalovirus (CMV). Individuals with PASLI disease also have an increased risk of EBV-associated lymphoma. Investigators Carrie Lucas, Michael Lenardo, and Gulbu Uzel at the National Institute of Allergy and Infectious Diseases at the U.S. National Institutes of Health and Sergey Nejentsev at the University of Cambridge, UK simultaneously described a mutation causing this condition, which they called activated PI3K delta syndrome (APDS). Signs and symptoms Clinically, PASLI disease is characterized by recurrent sinopulmonary infections that can lead to progressive airway damage. People also ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activated PI3K Delta Syndrome
Activated PI3K delta syndrome (APDS) is a primary immunodeficiency disease caused by activating gain of function mutations in the PIK3CD gene. Symptoms and signs The signs and symptoms of ''activated PI3K Delta Syndrome'' are consistent with the following: * Immunodeficiency * Lymphadenopathy * Sinopulmonary infections * Bronchiectasis Cause In terms of genetics, activated PI3K Delta Syndrome is autosomal dominant, a mutation in phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform is the reason for this condition (located at chromosome 1p36.) Mechanism The pathophysiology of activated PI3K delta syndrome has several aspects. The normal function has P110δ (PI3K) involved in immune system regulation. P110δ effect is not limited to the immune system; P110δ has a ''presence'' in transformed epithelial cells and cell adhesion molecules (airway inflammation), and research has been done on the possibility of P110δ in the nervous system. Activated PI3K ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leniolisib
Leniolisib (INN), sold under the brand name Joenja, is a medication used for the treatment of activated phosphoinositide 3-kinase delta syndrome (APDS). It is a kinase inhibitor that is taken by mouth. The most common side effects include headache, sinusitis, and atopic dermatitis. Leniolisib was approved for medical use in the United States in March 2023. It is the first approved medication for the treatment of activated PI3K delta syndrome. The US Food and Drug Administration (FDA) considers it to be a first-in-class medication. In 2025 NICE approved it for use in the British NHS. Medical uses Leniolisib is indicated for the treatment of activated phosphoinositide 3-kinase delta syndrome (activated PI3K delta syndrome) in people twelve years of age and older. Activated PI3K delta syndrome is caused by mutations in either of two genes, PIK3CD or PIK3R1, that regulate the maturation of white blood cells, especially B cells and T cells. This leads to a decrease in immun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marginal Zone Lymphoma
Marginal zone lymphomas, also known as marginal zone B-cell lymphomas (MZLs), are a heterogeneous group of lymphomas that derive from the malignant transformation of marginal zone B-cells. Marginal zone B cells are innate lymphoid cells that normally function by rapidly mounting IgM antibody immune responses to antigens such as those presented by infectious agents and damaged tissues. They are lymphocytes of the B-cell line that originate and mature in Lymphoid hyperplasia#Follicular hyperplasia, secondary lymphoid follicles and then move to the marginal zones of mucosa-associated lymphoid tissue (MALT), the spleen, or lymph nodes. Mucosa-associated lymphoid tissue is a diffuse system of small concentrations of lymphatic system#Lymphoid tissue, lymphoid tissue found in various mucosa, submucosal membrane sites of the human body, body such as the gastrointestinal tract, human mouth, mouth, nasal cavity, pharynx, thyroid gland, breast, human lung, lung, salivary glands, human eye, eye ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CSNK1E
Casein kinase I isoform epsilon is an enzyme that in humans is encoded by the ''CSNK1E'' gene. Function The protein encoded by this gene is a serine/threonine protein kinase and a member of the casein kinase I protein family, whose members have been implicated in the control of cytoplasmic and nuclear processes, including DNA replication and repair. The encoded protein is found in the cytoplasm as a monomer and can phosphorylate a variety of proteins, including itself. This protein has been shown to phosphorylate proteins of the Period family of circadian rhythm proteins. A homolog of this mammalian protein can be found in Drosophila melanogaster. Known as doubletime, this protein also plays a role in the phosphorylation of proteins involved in circadian rhythms. Two transcript variants encoding the same protein have been found for this gene. Interactions CSNK1E has been shown to interact with PER1 and AXIN1. Inhibitors ;Selective * PF-4800567 ;Non-selective * PF-670462 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Umbralisib
Umbralisib, sold under the brand name Ukoniq, is an anti-cancer medication for the treatment of marginal zone lymphoma (MZL) and follicular lymphoma (FL). It is taken by mouth. Umbralisib is a kinase inhibitor including PI3K-delta and casein kinase CK1-epsilon. The most common side effects include increased creatinine, diarrhea-colitis, fatigue, nausea, neutropenia, transaminase elevation, musculoskeletal pain, anemia, thrombocytopenia, upper respiratory tract infection, vomiting, abdominal pain, decreased appetite, and rash. Umbralisib was granted accelerated approval for medical use in the United States in February 2021. However, due to concerns for increased long term side effects leading to inferior overall survival which led to increased FDA scrutiny in the form of an ODAC review, it has been withdrawn from the US market. Medical uses In April 2022, TG Therapeutics announced the voluntary withdrawal of Ukoniq (umbralisib) from sale for its approved use in the treatment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fulvestrant
Fulvestrant, sold under the brand name Faslodex among others, is an antiestrogenic medication used to treat hormone receptor (HR)-positive metastatic breast cancer in postmenopausal women with disease progression as well as HR-positive, HER2-negative advanced breast cancer in combination with abemaciclib or palbociclib in women with disease progression after endocrine therapy. It is given by injection into a muscle. Fulvestrant is a selective estrogen receptor degrader (SERD) and was first-in-class to be approved. It works by binding to the estrogen receptor and destabilizing it, causing the cell's normal protein degradation processes to destroy it. Fulvestrant was approved for medical use in the United States in 2002. Medical uses Breast cancer Fulvestrant is used for the treatment of hormone receptor positive metastatic breast cancer or locally advanced unresectable disease in postmenopausal women; it is given by injection. A 2017 Cochrane review found it is as safe and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpelisib
Alpelisib, sold under the brand name Piqray among others, is a medication used to treat certain types of breast cancer. It is used together with fulvestrant. It is taken by mouth. It is marketed by Novartis. Common side effects include high blood sugar, kidney problems, diarrhea, rash, low blood cells, liver problems, pancreatitis, vomiting, and hair loss. It is an alpha-specific PI3K inhibitor. It was approved for medical use in the United States in May 2019. Medical uses Alpelisib is indicated in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer as detected by an FDA-approved test following progression on or after an endocrine-based regimen. In the European Union, alpelisib is indicated in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor (HR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Duvelisib
Duvelisib, sold under the brand name Copiktra, is a medication used to treat chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), and follicular lymphoma after other treatments have failed. It is taken by mouth. It is a PI3 kinase inhibitor. Common side effects include diarrhea, low white blood cells, rash, feeling tired, fever, and muscle pains. Other serious side effects include inflammation of the lungs and infections. It is a dual inhibitor of PI3Kδ and PI3Kγ. Medical uses Duvelisib is indicated to treat adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who have received at least two prior therapies that did not work or stopped working. CLL is a type of cancer that begins in the white blood cells, and SLL is a type of cancer that begins mostly in the lymph nodes. Adverse effects Duvelisib may cause infections, diarrhea, inflammation of the intestines and lungs, skin reactions, and high liver enzyme levels in the b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |