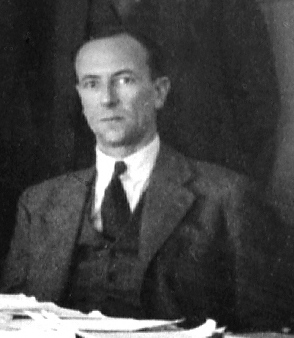|
Neutrons
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The neutron was discovered by James Chadwick in 1932, leading to the discovery of nuclear fission in 1938, the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity, 1945). Neutrons are found, together with a similar number of protons in the nuclei of atoms. Atoms of a chemical element that differ only in neutron number are called isotopes. Free neutrons are produced copiously in nuclear fission and fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes. Neutron stars, formed from massive collapsing stars, consist of neutrons at the density of atomic nuclei but a total mass more than the Sun. Neutron properties and interactions are described by nuclear physics. Neutrons are not elementary particles; each is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactive decay. Nuclear fission was discovered by chemists Otto Hahn and Fritz Strassmann and physicists Lise Meitner and Otto Robert Frisch. Hahn and Strassmann proved that a fission reaction had taken place on 19 December 1938, and Meitner and her nephew Frisch explained it theoretically in January 1939. Frisch named the process "fission" by analogy with biological fission of living cells. In their second publication on nuclear fission in February 1939, Hahn and Strassmann predicted the existence and liberation of additional neutrons during the fission process, opening up the possibility of a nuclear chain reaction. For heavy nuclides, it is an exothermic reaction which can release large amounts of energy both as electromagnetic radiat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Discovery Of The Neutron
The discovery of the neutron and its properties was central to the extraordinary developments in atomic physics in the first half of the 20th century. Early in the century, Ernest Rutherford developed a crude Rutherford model, model of the atom, based on the Geiger–Marsden experiment, gold foil experiment of Hans Geiger and Ernest Marsden. In this model, atoms had their mass and Electric charge, positive electric charge concentrated in a very small atomic nucleus, nucleus. By 1920, isotopes of chemical elements had been discovered, the atomic masses had been determined to be (approximately) Multiple (mathematics), integer multiples of the mass of the hydrogen atom, and the atomic number had been identified as the charge on the nucleus.Byrne, J. ''Neutrons, Nuclei, and Matter'', Dover Publications, Mineola, New York, 2011, Throughout the 1920s, the nucleus was viewed as composed of combinations of protons and electrons, the two elementary particles known at the time, but that mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Magnetic Moment
The nucleon magnetic moments are the intrinsic magnetic dipole moments of the proton and neutron, symbols ''μ''p and ''μ''n. The nucleus of an atom comprises protons and neutrons, both nucleons that behave as small magnets. Their magnetic strengths are measured by their magnetic moments. The nucleons interact with normal matter through either the nuclear force or their magnetic moments, with the charged proton also interacting by the Coulomb force. The proton's magnetic moment was directly measured in 1933 by Otto Stern team in University of Hamburg. While the neutron was determined to have a magnetic moment by indirect methods in the mid-1930s, Luis Alvarez and Felix Bloch made the first accurate, direct measurement of the neutron's magnetic moment in 1940. The proton's magnetic moment is exploited to make measurements of molecules by proton nuclear magnetic resonance. The neutron's magnetic moment is exploited to probe the atomic structure of materials using scatt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an electron (the proton-to-electron mass ratio). Protons and neutrons, each with a mass of approximately one Dalton (unit), dalton, are jointly referred to as ''nucleons'' (particles present in atomic nuclei). One or more protons are present in the Atomic nucleus, nucleus of every atom. They provide the attractive electrostatic central force which binds the atomic electrons. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol ''Z''). Since each chemical element, element is identified by the number of protons in its nucleus, each element has its own atomic number, which determines the number of atomic electrons and consequently the chemical characteristi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Neutron Decay
When embedded in an atomic nucleus, neutrons are (usually) stable particles. Outside the nucleus, free neutrons are unstable and have a mean lifetime of or (about and or , respectively). Therefore, the half-life for this process (which differs from the mean lifetime by a factor of ) is (about , ). The free neutron decays primarily by beta decay, with small probability of other channels. The beta decay of the neutron can be described at different levels of detail, starting with the simplest: : Quantitative measurements of the free neutron decay time vary slightly between different measurement techniques for reasons which have not been determined. Energy budget For the free neutron, the decay energy for this process (based on the rest masses of the neutron, proton and electron) is . That is the difference between the rest mass of the neutron and the sum of the rest masses of the products. That difference has to be carried away as kinetic energy. The maximal energy of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strong Interaction
In nuclear physics and particle physics, the strong interaction, also called the strong force or strong nuclear force, is one of the four known fundamental interaction, fundamental interactions. It confines Quark, quarks into proton, protons, neutron, neutrons, and other hadron particles, and also binds neutrons and protons to create atomic nuclei, where it is called the nuclear force. Most of the mass–energy equivalence, mass of a proton or neutron is the result of the strong interaction energy; the individual quarks provide only about 1% of the mass of a proton. At the range of 10−15 m (1 femtometer, slightly more than the radius of a nucleon), the strong force is approximately 100 times as strong as electromagnetism, 106 times as strong as the weak interaction, and 1038 times as strong as Gravity, gravitation. In the context of atomic nuclei, the force binds protons and neutrons together to form a nucleus and is called the nuclear force (or ''residual strong force'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
James Chadwick
Sir James Chadwick (20 October 1891 – 24 July 1974) was an English nuclear physicist who received the Nobel Prize in Physics in 1935 for his discovery of the neutron. In 1941, he wrote the final draft of the MAUD Report, which inspired the U.S. government to begin serious atomic bomb research efforts. He was the head of the British team that worked on the Manhattan Project during World War II. He was knighted in Britain in 1945 for his achievements in nuclear physics. Chadwick graduated from the Victoria University of Manchester in 1911, where he studied under Ernest Rutherford (known as the "father of nuclear physics"). At Manchester, he continued to study under Rutherford until he was awarded his MSc in 1913. The same year, Chadwick was awarded an 1851 Research Fellowship from the Royal Commission for the Exhibition of 1851. He elected to study beta radiation under Hans Geiger in Berlin. Using Geiger's recently developed Geiger counter, Chadwick was able to demons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hadron
In particle physics, a hadron is a composite subatomic particle made of two or more quarks held together by the strong nuclear force. Pronounced , the name is derived . They are analogous to molecules, which are held together by the electric force. Most of the mass of ordinary matter comes from two hadrons: the proton and the neutron, while most of the mass of the protons and neutrons is in turn due to the binding energy of their constituent quarks, due to the strong force. Hadrons are categorized into two broad families: baryons, made of an odd number of quarks (usually three) and mesons, made of an even number of quarks (usually two: one quark and one antiquark). Protons and neutrons (which make the majority of the mass of an atom) are examples of baryons; pions are an example of a meson. A tetraquark state (an exotic meson), named the Z(4430), was discovered in 2007 by the Belle Collaboration and confirmed as a resonance in 2014 by the LHCb collaboration. Two pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Subatomic Particle
In physics, a subatomic particle is a particle smaller than an atom. According to the Standard Model of particle physics, a subatomic particle can be either a composite particle, which is composed of other particles (for example, a baryon, like a proton or a neutron, composed of three quarks; or a meson, composed of two quarks), or an elementary particle, which is not composed of other particles (for example, quarks; or electrons, muons, and tau particles, which are called leptons). Particle physics and nuclear physics study these particles and how they interact. Most force-carrying particles like photons or gluons are called bosons and, although they have quanta of energy, do not have rest mass or discrete diameters (other than pure energy wavelength) and are unlike the former particles that have rest mass and cannot overlap or combine which are called fermions. The W and Z bosons, however, are an exception to this rule and have relatively large rest masses at approxim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antineutron
The antineutron is the antiparticle of the neutron with symbol . It differs from the neutron only in that some of its properties have equal magnitude but opposite sign. It has the same mass as the neutron, and no net electric charge, but has opposite baryon number (+1 for neutron, −1 for the antineutron). This is because the antineutron is composed of antiquarks, while neutrons are composed of quarks. The antineutron consists of one up antiquark and two down antiquarks. Background The antineutron was discovered in proton–antiproton collisions at the Bevatron (Lawrence Berkeley National Laboratory) by the team of Bruce Cork, Glen Lambertson, Oreste Piccioni, and William Wenzel in 1956, one year after the antiproton was discovered. Since the antineutron is electrically neutral, it cannot easily be observed directly. Instead, the products of its annihilation with ordinary matter are observed. In theory, a free antineutron should decay into an antiproton, a positron, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isospin
In nuclear physics and particle physics, isospin (''I'') is a quantum number related to the up- and down quark content of the particle. Isospin is also known as isobaric spin or isotopic spin. Isospin symmetry is a subset of the flavour symmetry seen more broadly in the interactions of baryons and mesons. The name of the concept contains the term ''spin'' because its quantum mechanical description is mathematically similar to that of angular momentum (in particular, in the way it couples; for example, a proton–neutron pair can be coupled either in a state of total isospin 1 or in one of 0). But unlike angular momentum, it is a dimensionless quantity and is not actually any type of spin. Before the concept of quarks was introduced, particles that are affected equally by the strong force but had different charges (e.g. protons and neutrons) were considered different states of the same particle, but having isospin values related to the number of charge states. A close exami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quark Structure Neutron
A quark () is a type of elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. All commonly observable matter is composed of up quarks, down quarks and electrons. Owing to a phenomenon known as '' color confinement'', quarks are never found in isolation; they can be found only within hadrons, which include baryons (such as protons and neutrons) and mesons, or in quark–gluon plasmas. There is also the theoretical possibility of more exotic phases of quark matter. For this reason, much of what is known about quarks has been drawn from observations of hadrons. Quarks have various intrinsic properties, including electric charge, mass, color charge, and spin. They are the only elementary particles in the Standard Model of particle physics to experience all four fundamental interactions, also known as ''fundamental forces'' (ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |