|
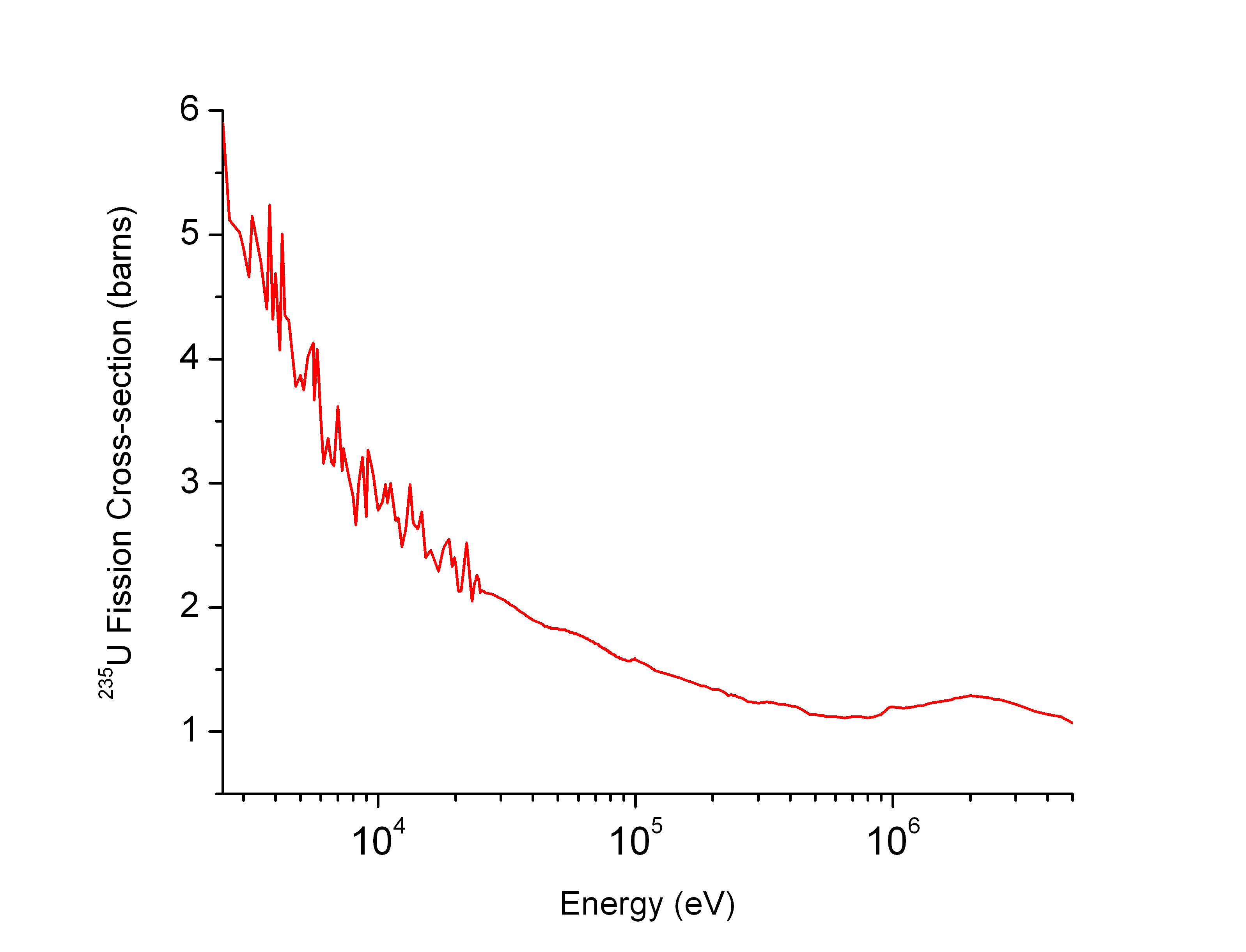
Neutron Cross Section
In nuclear physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of neutron-nuclei reactions taking place is equal to the product of the number of incident neutrons that would pass through the area and the number of target nuclei. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power of a nuclear power plant. The standard unit for measuring the cross section is the barn, which is equal to 10−28 m2 or 10−24 cm2. The larger the neutron cross section, the more likely a neutron will react with the nucleus. An isotope (or nuclide) can be classified according to its neutron cross section and how it reacts to an incident neutron. Nuclides that tend to absorb a neutron and either decay or keep the neutron in its nucleus are neutron a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter. Nuclear physics should not be confused with atomic physics, which studies the atom as a whole, including its electrons. Discoveries in nuclear physics have led to applications in many fields such as nuclear power, nuclear weapons, nuclear medicine and magnetic resonance imaging, industrial and agricultural isotopes, ion implantation in materials engineering, and radiocarbon dating in geology and archaeology. Such applications are studied in the field of nuclear engineering. Particle physics evolved out of nuclear physics and the two fields are typically taught in close association. Nuclear astrophysics, the application of nuclear physics to astrophysics, is crucial in explaining the inner workings of stars and the origin of the chemical elements. History The history of nuclear physics as a discipline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
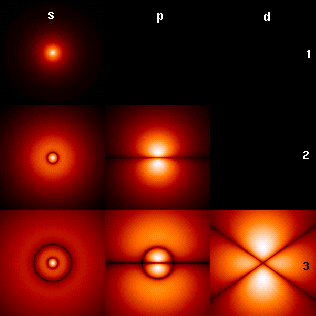
Spin (physics)
Spin is an Intrinsic and extrinsic properties, intrinsic form of angular momentum carried by elementary particles, and thus by List of particles#Composite particles, composite particles such as hadrons, atomic nucleus, atomic nuclei, and atoms. Spin is quantized, and accurate models for the interaction with spin require relativistic quantum mechanics or quantum field theory. The existence of electron spin angular momentum is inferred from experiments, such as the Stern–Gerlach experiment, in which silver atoms were observed to possess two possible discrete angular momenta despite having no orbital angular momentum. The relativistic spin–statistics theorem connects electron spin quantization to the Pauli exclusion principle: observations of exclusion imply half-integer spin, and observations of half-integer spin imply exclusion. Spin is described mathematically as a vector for some particles such as photons, and as a spinor or bispinor for other particles such as electrons. Sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scattering
In physics, scattering is a wide range of physical processes where moving particles or radiation of some form, such as light or sound, are forced to deviate from a straight trajectory by localized non-uniformities (including particles and radiation) in the medium through which they pass. In conventional use, this also includes deviation of reflected radiation from the angle predicted by the law of reflection. Reflections of radiation that undergo scattering are often called ''diffuse reflections'' and unscattered reflections are called ''specular'' (mirror-like) reflections. Originally, the term was confined to light scattering (going back at least as far as Isaac Newton in the 17th century). As more "ray"-like phenomena were discovered, the idea of scattering was extended to them, so that William Herschel could refer to the scattering of "heat rays" (not then recognized as electromagnetic in nature) in 1800. John Tyndall, a pioneer in light scattering research, noted the connecti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Protactinium
Protactinium (91Pa) has no stable isotopes. The four naturally occurring isotopes allow a standard atomic weight to be given. Thirty radioisotopes of protactinium have been characterized, ranging from 210Pa to 239Pa. The most stable isotope is 231Pa with a half-life of 32,760 years, 233Pa with a half-life of 26.967 days, and 230Pa with a half-life of 17.4 days. All of the remaining radioactive isotopes have half-lives less than 1.6 days, and the majority of these have half-lives less than 1.8 seconds. This element also has five meta states, 217mPa (t1/2 1.15 milliseconds), 220m1Pa (t1/2 = 308 nanoseconds), 220m2Pa (t1/2 = 69 nanoseconds), 229mPa (t1/2 = 420 nanoseconds), and 234mPa (t1/2 = 1.17 minutes). The only naturally occurring isotopes are 231Pa, 234Pa and 234mPa. The former occurs as an intermediate decay product of 235U, while the latter two occur as intermediate decay products of 238U. 231Pa makes up nearly all natural protactinium. The primary de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CANDU Reactor
The CANDU (CANada Deuterium Uranium) is a Canadian pressurized heavy-water reactor design used to generate electric power. The acronym refers to its deuterium oxide (heavy water) moderator and its use of (originally, natural) uranium fuel. CANDU reactors were first developed in the late 1950s and 1960s by a partnership between Atomic Energy of Canada Limited (AECL), the Hydro-Electric Power Commission of Ontario, Canadian General Electric, and other companies. There have been two major types of CANDU reactors, the original design of around 500 MWe that was intended to be used in multi-reactor installations in large plants, and the optimized CANDU 6 in the 600 MWe class that is designed to be used in single stand-alone units or in small multi-unit plants. CANDU 6 units were built in Quebec and New Brunswick, as well as Pakistan, Argentina, South Korea, Romania, and China. A single example of a non-CANDU 6 design was sold to India. The multi-unit design was used onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enriched Uranium
Enriched uranium is a type of uranium in which the percent composition of uranium-235 (written 235U) has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238 (238U with 99.2732–99.2752% natural abundance), uranium-235 (235U, 0.7198–0.7210%), and uranium-234 (234U, 0.0049–0.0059%). 235U is the only nuclide existing in nature (in any appreciable amount) that is fissile with thermal neutrons. Enriched uranium is a critical component for both civil nuclear power generation and military nuclear weapons. Low-enriched uranium (20% 235U, typically >85%) is used for the cores of many nuclear weapons, as well as compact reactors for naval propulsion and research, as well as breeder reactors. There are about 2,000 tonnes of highly enriched uranium in the world. Enrichment methods were first developed on a large scale by the Manhattan Project. Its gaseous diffusion method was used in the 194 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Uranium
Natural uranium (NU or Unat) is uranium with the same isotopic ratio as found in nature. It contains 0.711% uranium-235, 99.284% uranium-238, and a trace of uranium-234 by weight (0.0055%). Approximately 2.2% of its radioactivity comes from uranium-235, 48.6% from uranium-238, and 49.2% from uranium-234. Natural uranium can be used to fuel both low- and high-power nuclear reactors. Historically, graphite-moderated reactors and heavy water-moderated reactors have been fueled with natural uranium in the pure metal (U) or uranium dioxide (UO2) ceramic forms. However, experimental fuelings with uranium trioxide (UO3) and triuranium octaoxide (U3O8) have shown promise. The 0.72% uranium-235 is not sufficient to produce a self-sustaining critical chain reaction in light water reactors or nuclear weapons; these applications must use enriched uranium. Nuclear weapons take a concentration of 90% uranium-235, and light water reactors require a concentration of roughly 3% uranium-235 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Light Water Reactor
The light-water reactor (LWR) is a type of thermal-neutron reactor that uses normal water, as opposed to heavy water, as both its coolant and neutron moderator; furthermore a solid form of fissile elements is used as fuel. Thermal-neutron reactors are the most common type of nuclear reactor, and light-water reactors are the most common type of thermal-neutron reactor. There are three varieties of light-water reactors: the pressurized water reactor (PWR), the boiling water reactor (BWR), and (most designs of) the supercritical water reactor (SCWR). History Early concepts and experiments After the discoveries of fission, moderation and of the theoretical possibility of a nuclear chain reaction, early experimental results rapidly showed that natural uranium could only undergo a sustained chain reaction using graphite or heavy water as a moderator. While the world's first reactors ( CP-1, X10 etc.) were successfully reaching criticality, uranium enrichment began to devel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heavy Water Reactor
A heavy water reactor (HWR) is a type of nuclear reactor which uses heavy water (D2O, deuterium oxide) as a neutron moderator. It may also use this as the coolant, in the case of pressurized heavy water reactors. Due to heavy water's low neutron absorption cross section, HWRs can operate with natural uranium fuel. History "Atomic pile" experiments were carried out across Europe and North America following the 1938 discovery of nuclear fission. The sole supply of heavy water was from the Vemork hydroelectric power plant in Norway. The world's entire supply had been sent to Paris for experiments, but smuggled to England during the Fall of France. In November 1940, Hans von Halban and Lew Kowarski at the University of Cambridge carried out one of the first heavy water-moderated pile experiments, measuring net neutron production. In 1958, Patrick Blackett wrote about Frédéric Joliot-Curie: "There is little doubt that, had the war not intervened, the world’s first self-sustain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral hydrogen atom contains a single positively charged proton in the nucleus, and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe. In everyday life on Earth, isolated hydrogen atoms (called "atomic hydrogen") are extremely rare. Instead, a hydrogen atom tends to combine with other atoms in compounds, or with another hydrogen atom to form ordinary (diatomic) hydrogen gas, H2. "Atomic hydrogen" and "hydrogen atom" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms). Atomic spectroscopy shows that there is a discrete infinite set of states in which a hydrogen (or any) atom can exist, contrary to the predictions of classical physics. At ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium nucleus (deuteron) contains one proton and one neutron, whereas the far more common H has no neutrons. The name ''deuterium'' comes from Greek '' deuteros'', meaning "second". American chemist Harold Urey discovered deuterium in 1931. Urey and others produced samples of heavy water in which the H had been highly concentrated. The discovery of deuterium won Urey a Nobel Prize in 1934. Nearly all deuterium found in nature was synthesized in the Big Bang 13.8 billion years ago, forming the primordial ratio of H to H (~26 deuterium nuclei per 10 hydrogen nuclei). Deuterium is subsequently produced by the slow stellar proton–proton chain, but rapidly destroyed by exothermic fusion reactions. The deuterium–deuterium reaction has the second-lowest energy threshold, and is the most astrophysically acce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |