|
NOx
In atmospheric chemistry, is shorthand for nitric oxide () and nitrogen dioxide (), the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone. gases are usually produced from the reaction between nitrogen and oxygen during combustion of fuels, such as hydrocarbons, in air; especially at high temperatures, such as in car engines. In areas of high motor vehicle traffic, such as in large cities, the nitrogen oxides emitted can be a significant source of air pollution. gases are also produced naturally by lightning. does not include nitrous oxide (), a fairly inert oxide of nitrogen that contributes less severely to air pollution, notwithstanding its involvement in ozone depletion and high global warming potential. is the class of compounds comprising and the compounds produced from the oxidation of which include nitric acid, nitrous acid (HONO), dinitr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion (e.g., using a lit match to light a fire), the heat from a flame may provide enough energy to make the reaction self-sustaining. The study of combustion is known as combustion science. Combustion is often a complicated sequence of elementary reaction, elementary Radical (chemistry), radical reactions. Solid fuels, such as wood and coal, first undergo endothermic pyrolysis to produce gaseous fuels whose combustion then supplies the heat required to produce more of them. Combustion is often hot e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tropospheric Ozone
Ground-level ozone (), also known as surface-level ozone and tropospheric ozone, is a trace gas in the troposphere (the lowest level of the Earth's atmosphere), with an average concentration of 20–30 parts per billion by volume (ppbv), with close to 100 ppbv in polluted areas. Ozone is also an important constituent of the stratosphere, where the ozone layer (2 to 8 parts per million ozone) exists which is located between 10 and 50 kilometers above the Earth's surface. The troposphere extends from the ground up to a variable height of approximately 14 kilometers above sea level. Ozone is least concentrated in the ground layer (or planetary boundary layer) of the troposphere. Ground-level or tropospheric ozone is created by chemical reactions between NOx gases (oxides of nitrogen produced by combustion) and volatile organic compounds (VOCs). The combination of these chemicals in the presence of sunlight form ozone. Its concentration increases as height above sea level increases, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Lightning
Lightning is a natural phenomenon consisting of electrostatic discharges occurring through the atmosphere between two electrically charged regions. One or both regions are within the atmosphere, with the second region sometimes occurring on the land, ground. Following the lightning, the regions become partially or wholly electrically neutralized. Lightning involves a near-instantaneous release of energy on a scale averaging between 200 megajoules and 7 gigajoules. The air around the lightning flash rapidly heats to temperatures of about . There is an emission of electromagnetic radiation across a wide range of wavelengths, some visible as a bright flash. Lightning also causes thunder, a sound from the shock wave which develops as heated gases in the vicinity of the discharge experience a sudden increase in pressure. The most common occurrence of a lightning event is known as a thunderstorm, though they can also commonly occur in other types of energetic weather systems, such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lower atmosphere to (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the atmosphere, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation. Ozone's odor is reminiscent of chlorine, and detectable by many people at concentrations of as little as in air. Ozone's O3 chemical structure, structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetism, diamagnetic. At standard temperature and pressure, ozone is a pale blue gas that condenses at cryogenic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Air Pollution
Air pollution is the presence of substances in the Atmosphere of Earth, air that are harmful to humans, other living beings or the environment. Pollutants can be Gas, gases like Ground-level ozone, ozone or nitrogen oxides or small particles like soot and dust. It affects both outdoor air and indoor air. Natural sources of air pollution include Wildfire, wildfires, Dust storm, dust storms, and Volcanic eruption, volcanic eruptions. Indoor air pollution is often Energy poverty and cooking, caused by the use of biomass (e.g. wood) for cooking and heating. Outdoor air pollution comes from some industrial processes, the burning of Fossil fuel, fossil fuels for electricity and transport, waste management and agriculture. Many of the contributors of local air pollution, especially the burning of fossil fuels, also cause greenhouse gas emissions that cause climate change, global warming. Air pollution causes around 7 or 8 million deaths each year. It is a significant risk factor for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
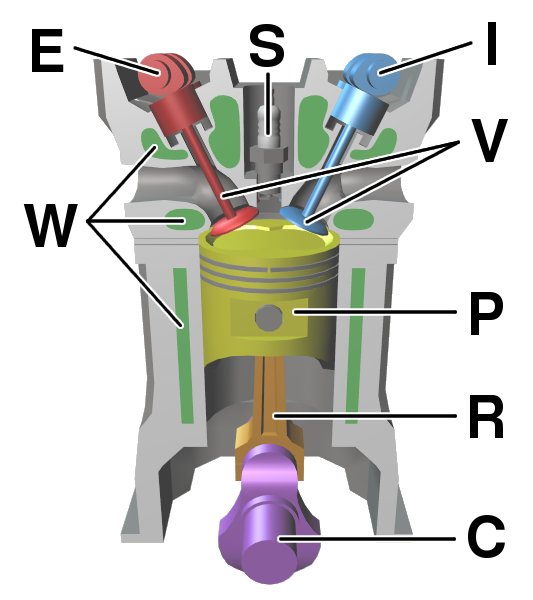
Internal Combustion Engine
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combustion engine, the expansion of the high-temperature and high-pressure gases produced by combustion applies direct force to some component of the engine. The force is typically applied to pistons (reciprocating engine, piston engine), turbine blades (gas turbine), a Wankel engine, rotor (Wankel engine), or a propulsive nozzle, nozzle (jet engine). This force moves the component over a distance. This process transforms chemical energy into kinetic energy which is used to propel, move or power whatever the engine is attached to. The first commercially successful internal combustion engines were invented in the mid-19th century. The first modern internal combustion engine, the Otto engine, was designed in 1876 by the German engineer Nicolaus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrogen Dioxide
Nitrogen dioxide is a chemical compound with the formula . One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers. Nitrogen dioxide is poisonous and can be fatal if inhaled in large quantities. Cooking with a gas stove produces nitrogen dioxide which causes poorer indoor air quality. Combustion of gas can lead to increased concentrations of nitrogen dioxide throughout the home environment which is linked to respiratory issues and diseases. The LC50 ( median lethal dose) for humans has been estimated to be 174 ppm for a 1-hour exposure. It is also included in the NOx family of atmospheric pollutants. Properties Nitrogen dioxide is a reddish-brown gas with a pungent, acrid odor above and becomes a yellowish-brown liquid below . ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitric Oxide
Nitric oxide (nitrogen oxide, nitrogen monooxide, or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (•N=O or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding. An important intermediate in industrial chemistry, nitric oxide forms in combustion systems and can be generated by lightning in thunderstorms. In mammals, including humans, nitric oxide is a signaling molecule in many physiological and pathological processes. It was proclaimed the " Molecule of the Year" in 1992. The 1998 Nobel Prize in Physiology or Medicine was awarded for discovering nitric oxide's role as a cardiovascular signalling molecule. Its impact extends beyond biology, with applications in medicine, such as the development of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acid Rain
Acid rain is rain or any other form of Precipitation (meteorology), precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists between 6.5 and 8.5, but acid rain has a pH level lower than this and ranges from 4–5 on average. The more acidic the acid rain is, the lower its pH is. Acid rain can have harmful effects on plants, aquatic animals, and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the Properties of water, water molecules in the atmosphere to produce acids. Acid rain has been shown to have adverse impacts on forests, Fresh water, freshwaters, soils, microbes, insects and aquatic life-forms. In ecosystems, persistent acid rain reduces tree bark durability, leaving flora more susceptible to environmental stressors such as drought, heat/cold and pest infestation. Acid rain is also capable of detriment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Smog
Smog, or smoke fog, is a type of intense air pollution. The word "smog" was coined in the early 20th century, and is a portmanteau of the words ''smoke'' and ''fog'' to refer to smoky fog due to its opacity, and odour. The word was then intended to refer to what was sometimes known as pea soup fog, a familiar and serious problem in London from the 19th century to the mid-20th century, where it was commonly known as a London particular or London fog. This kind of visible air pollution is composed of nitrogen oxides, sulfur oxide, ozone, smoke and other Particulate matter, particulates. Man-made smog is derived from coal combustion emissions, vehicular emissions, industrial emissions, forest and agricultural fires and photochemical reactions of these emissions. Smog is often categorized as being either summer smog or winter smog. Summer smog is primarily associated with the photochemical formation of ozone. During the summer season when the temperatures are warmer and there is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrogen Oxide
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds: Charge-neutral *Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide * Nitrogen dioxide (), nitrogen(IV) oxide * Nitrogen trioxide (), or nitrate radical *Nitrous oxide (), nitrogen(0,II) oxide * Dinitrogen dioxide (), nitrogen(II) oxide dimer * Dinitrogen trioxide (), nitrogen(II,IV) oxide * Dinitrogen tetroxide (), nitrogen(IV) oxide dimer *Dinitrogen pentoxide (), nitrogen(V) oxide, or nitronium nitrate * Nitrosyl azide (), nitrogen(−I,0,I,II) oxide * Nitryl azide () * Oxatetrazole () * Trinitramide ( or ), nitrogen(0,IV) oxide Anions Cations * Nitrosonium ( or ) * Nitronium ( or ) Atmospheric sciences In atmospheric chemistry: * (or NO''x'') refers to the sum of NO and . * (or NO''y'') refers to the sum of and all oxidized atmospheric odd-nitrogen species (''e.g.'' the sum of , , , etc.) * (or NO''z'') = − * Mixed Oxides of Nitrogen ("MON"): solu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrous Oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or factitious air, among others, is a chemical compound, an Nitrogen oxide, oxide of nitrogen with the Chemical formula, formula . At room temperature, it is a colourless Flammability#Definitions, non-flammable gas, and has a slightly sweet scent and taste. At elevated temperatures, nitrous oxide is a powerful Oxidising agent, oxidiser similar to molecular oxygen. Nitrous oxide has significant Nitrous oxide (medication), medical uses, especially in surgery and dentistry, for its Anesthesia, anaesthetic and Analgesic, pain-reducing effects, and it is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. Its colloquial name, "laughing gas", coined by Humphry Davy, describes the Euphoria, euphoric effects upon inhaling it, which cause it to be used as a recreational drug inducing a brief "Dissociative, high". When abused chronically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |