|
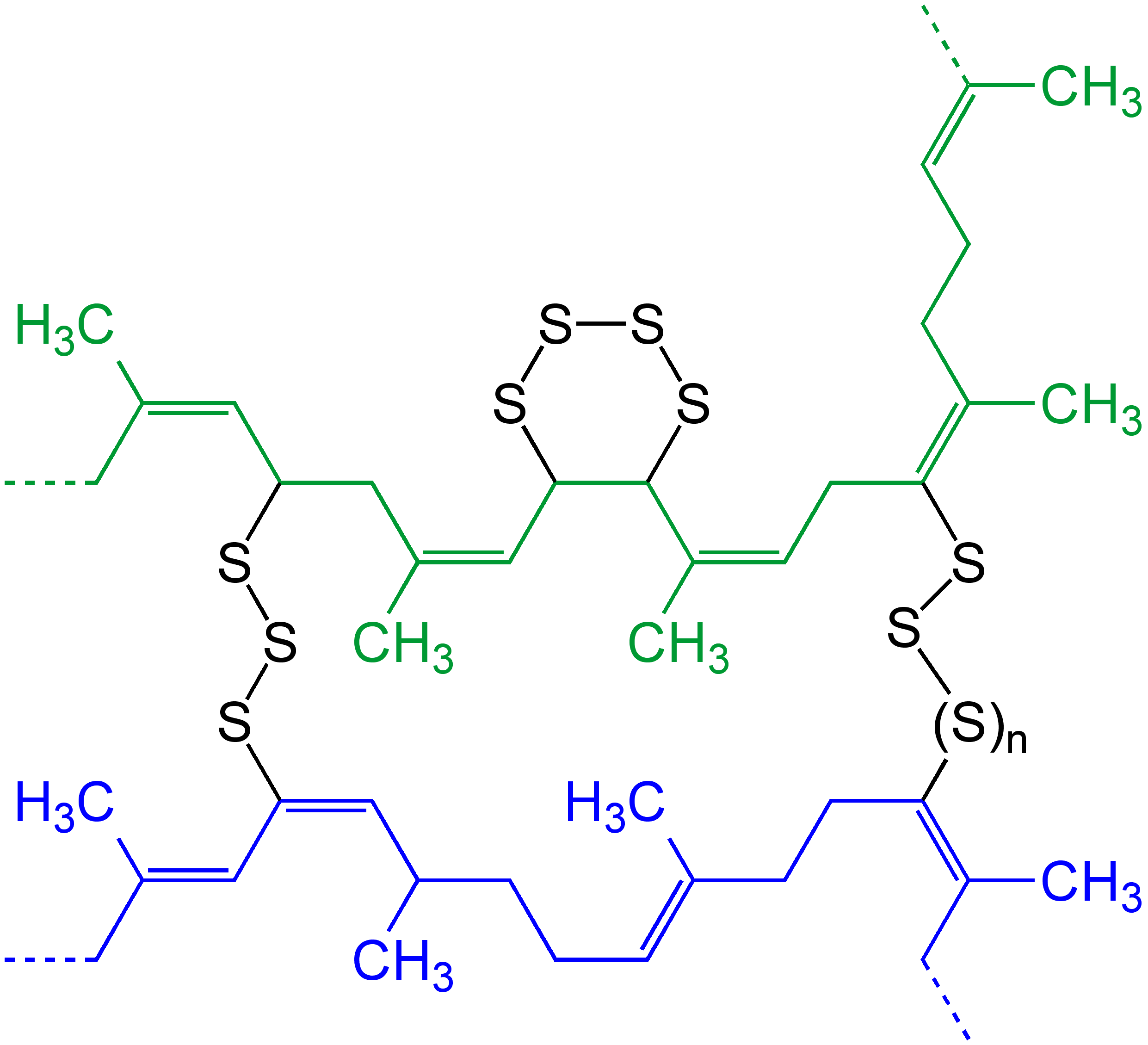
N,N'-Diallyl-L-tartardiamide
''N'',''N''′-Diallyl-L-tartardiamide (DATD) is a crosslinking agent for polyacrylamide gels, e.g., as used for SDS-PAGE. Compared to N,N'-Methylenebisacrylamide, bisacrylamide gels, DATD gels have a stronger interaction with glass, and therefore are used in applications where the polyacrylamide gel acts as a "plug" structural component at the bottom of a gel electrophoresis apparatus, thereby preventing a weak discontinuous gel from sliding out from or otherwise moving within the apparatus. Unlike bisacrylamide-polyacrylamide gels, DATD-polyacrylamide gels can be conveniently dissolved using periodic acid due to the presence of Diol#Vicinal diols, viscinal diols in DATD. DATD is the slowest polyacrylamide crosslinker tested, and has can act as an inhibitor of polymerization at high concentrations. See also * N,N'-Methylenebisacrylamide, bisacrylamide References Acrylamides Monomers Diols {{alkene-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N,N'-Methylenebisacrylamide
''N'',''N''′-Methylenebisacrylamide (MBAm or MBAA, colloquially "''bis''") is the organic compound with the formula CH2[NHC(O)CH=CH2]2. A colorless solid, this compound is a crosslinking agent in polyacrylamides, e.g., as used for SDS-PAGE. Synthesis Acrylamide reacts with an aqueous solution of formaldehyde in the presence of copper(I) chloride as a polymerization inhibitor and sulfuric acid as catalyst to form ''N'',''N''′-methylenebisacrylamide with yields of 60 to 80%. The reaction proceeds via ''N''-hydroxymethylacrylamide, which can be detected in alkaline solution and decomposes in acid to give ''N'',''N''′-methylenebisacrylamide. Using acrylamide and paraformaldehyde in 1,2-dichloroethane gives a clear solution upon heating, from which MBA crystallizes. In aqueous media, acrylonitrile also reacts with formaldehyde to give crude ''N'',''N''′-methylenebisacrylamide, which can be purified by recrystallization with acetone/water. Reactions and uses Under basic c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crosslinking Agent
In chemistry and biology, a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural polymers (such as proteins). In polymer chemistry "cross-linking" usually refers to the use of cross-links to promote a change in the polymers' physical properties. When "crosslinking" is used in the biological field, it refers to the use of a probe to link proteins together to check for protein–protein interactions, as well as other creative cross-linking methodologies. Although the term is used to refer to the "linking of polymer chains" for both sciences, the extent of crosslinking and specificities of the crosslinking agents vary greatly. Synthetic polymers : 260px, left, Chemical reactions associated with crosslinking of linoleum.html" ;"title="drying oils, the process that produces linoleum">drying oils, the process that produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyacrylamide
Polyacrylamide (abbreviated as PAM or pAAM) is a polymer with the formula (-CH2CHCONH2-). It has a linear-chain structure. PAM is highly water-absorbent, forming a soft gel when hydrated. In 2008, an estimated 750,000,000 kg were produced, mainly for water treatment and the paper and mineral industries. Physicochemical properties Polyacrylamide is a polyolefin. It can be viewed as polyethylene with amide substituents on alternating carbons. Unlike various nylons, polyacrylamide is not a polyamide because the amide groups are not in the polymer backbone. Owing to the presence of the amide (CONH2) groups, alternating carbon atoms in the backbone are stereogenic (colloquially: chiral). For this reason, polyacrylamide exists in atactic, syndiotactic, and isotactic forms, although this aspect is rarely discussed. The polymerization is initiated with radicals and is assumed to be stereorandom. Copolymers and modified polymers Linear polyacrylamide is a water-soluble polymer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
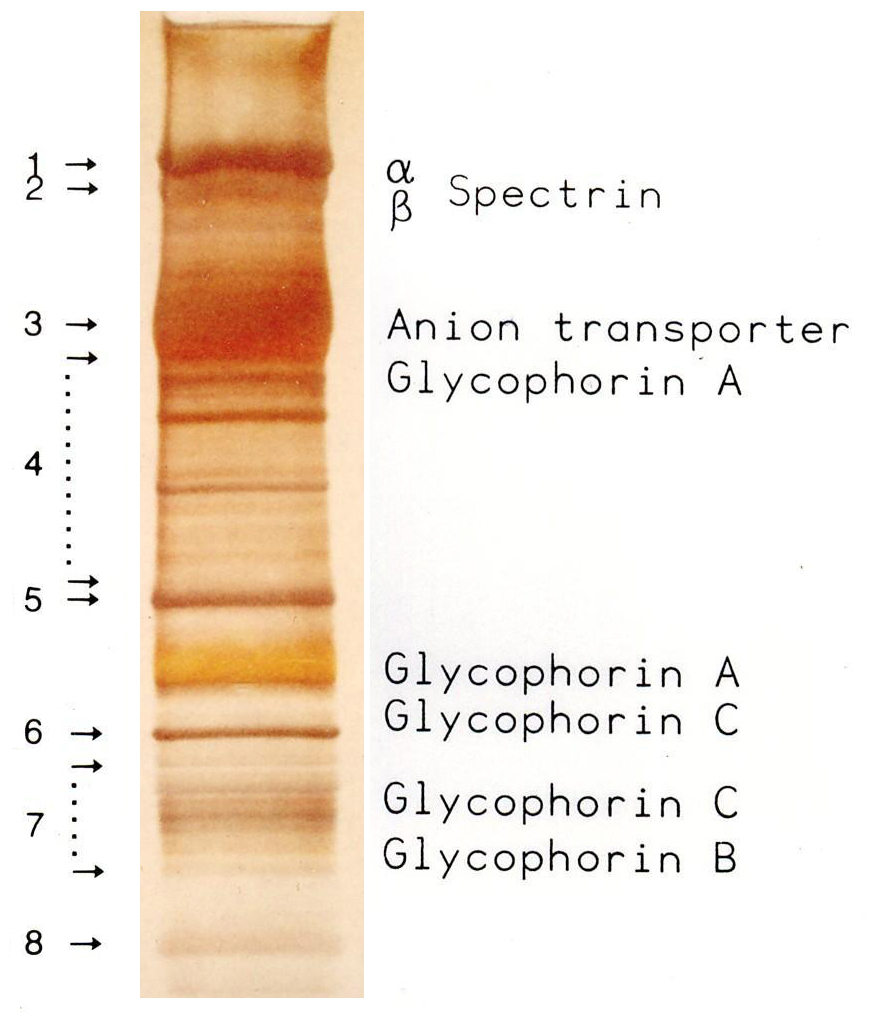
SDS-PAGE
SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a Discontinuous electrophoresis, discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 Kilodalton, kDa. The combined use of sodium dodecyl sulfate (SDS, also known as sodium lauryl sulfate) and polyacrylamide gel eliminates the influence of structure and charge, and proteins are separated by differences in their size. At least up to 2012, the publication describing it was the most frequently cited paper by a single author, and the second most cited overall. Properties SDS-PAGE is an electrophoresis method that allows protein separation by mass. The medium (also referred to as ′matrix′) is a polyacrylamide-based discontinuous gel. The polyacrylamide-gel is typically sandwiched between two glass plates in a slab gel. Although tube gels (in glass cylinders) were used historically, they were rapid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass are named after the material, e.g., a Tumbler (glass), "glass" for drinking, "glasses" for vision correction, and a "magnifying glass". Glass is most often formed by rapid cooling (quenching) of the Melting, molten form. Some glasses such as volcanic glass are naturally occurring, and obsidian has been used to make arrowheads and knives since the Stone Age. Archaeological evidence suggests glassmaking dates back to at least 3600 BC in Mesopotamia, Ancient Egypt, Egypt, or Syria. The earliest known glass objects were beads, perhaps created accidentally during metalworking or the production of faience, which is a form of pottery using lead glazes. Due to its ease of formability int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
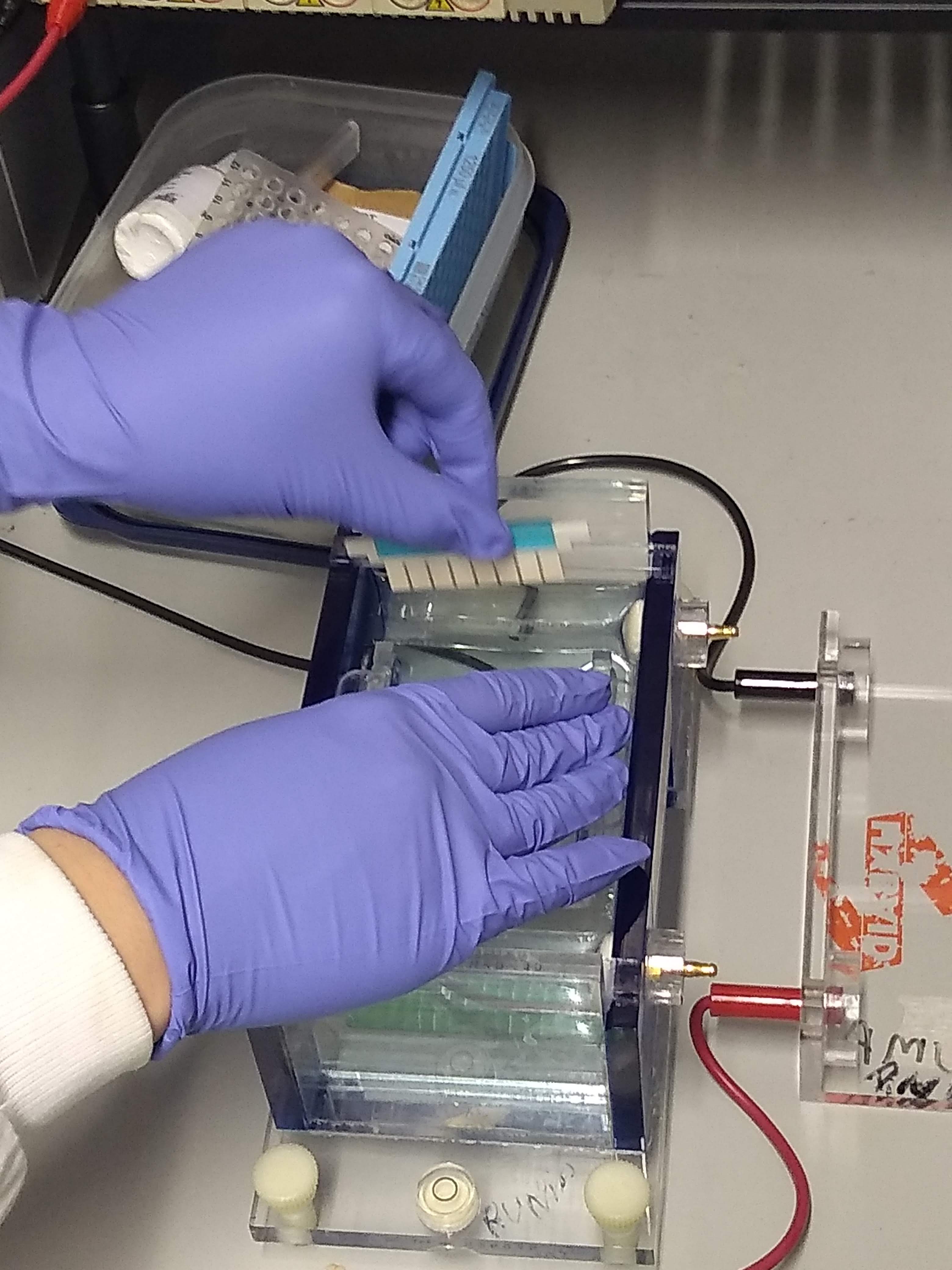
Gel Electrophoresis
Gel electrophoresis is an electrophoresis method for separation and analysis of biomacromolecules (DNA, RNA, proteins, etc.) and their fragments, based on their size and charge through a gel. It is used in clinical chemistry to separate proteins by charge or size (IEF agarose, essentially size independent) and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments, or to separate proteins by charge. Nucleic acid molecules are separated by applying an electric field to move the negatively charged molecules through a gel matrix of agarose, polyacrylamide, or other substances. Shorter molecules move faster and migrate farther than longer ones because shorter molecules migrate more easily through the pores of the gel. This phenomenon is called sieving. Proteins are separated by the charge in agarose because the pores of the gel are too large to sieve proteins. Gel electrophoresi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodic Acid
Periodic acid ( ) is an oxoacid of iodine. It can exist in two forms: orthoperiodic acid, with the chemical formula , and metaperiodic acid, which has the formula . Periodic acids are colourless crystals. Periodic acid features iodine in the highest oxidation state of +7. Periodic acid was discovered by Heinrich Gustav Magnus and C. F. Ammermüller in 1833. Synthesis Modern industrial scale production involves the oxidation of a solution of sodium iodate under Alkali, alkaline conditions, either electrochemically on a Lead dioxide, anode, or by treatment with chlorine: : (counter ions omitted for clarity) Standard electrode potential, ''E''° = −1.6 V : A standard laboratory preparation involves treating a mixture of tribarium dihydrogen orthoperiodate with nitric acid. Upon concentrating the mixture, the barium nitrate, which is less soluble, is separated from periodic acid: : Properties Orthoperiodic acid has a number of acid dissociation constants. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting groups of carbonyl groups, making them essential in synthesis of organic chemistry. The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol and propylene-1,3-diol, or beta propylene glycol, . Synthesis of classes of diols Geminal diols A geminal diol has two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. The hydration is usually unfavorable, but a notable exception is formaldehyde which, in water, exists in equilibrium with methanediol H2C(OH)2. Another example is (F3C)2C(OH)2, the hydrated form of hexafluoroacetone. Many gem-diols undergo further condensation to give dimer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrylamides
Acrylamide (or acrylic amide) is an organic compound with the chemical formula CH2=CHC(O)NH2. It is a white odorless solid, soluble in water and several organic solvents. From the chemistry perspective, acrylamide is a vinyl-substituted primary amide (CONH2). It is produced industrially mainly as a precursor to polyacrylamides, which find many uses as water-soluble thickeners and flocculation agents. Acrylamide forms in burnt areas of food, particularly starchy foods like potatoes, when cooked with high heat, above . Despite health scares following this discovery in 2002, and its classification as a probable carcinogen, acrylamide from diet is thought unlikely to cause cancer in humans; Cancer Research UK categorized the idea that eating burnt food causes cancer as a "myth". Production Acrylamide can be prepared by the hydration of acrylonitrile, which is catalyzed enzymatically: :CH2=CHCN + H2O → CH2=CHC(O)NH2 This reaction also is catalyzed by sulfuric acid as well as v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomers
A monomer ( ; ''wikt:mono-, mono-'', "one" + ''wikt:-mer, -mer'', "part") is a molecule that can chemical reaction, react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization. Classification Chemistry classifies monomers by type, and two broad classes based on the type of polymer they form. By type: * natural vs synthetic, e.g. glycine vs caprolactam, respectively * polar vs nonpolar, e.g. vinyl acetate vs ethylene, respectively * cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively By type of polymer they form: * those that participate in condensation polymerization * those that participate in addition polymerization Differing stoichiometry causes each class to create its respective form of polymer. : The polymerization of one kind of monomer gives a polymer#Monomers and repeat units, homopolymer. Many polymers are copolymers, meaning that they are derived from two diff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |