|
Metallophilic Interaction
In chemistry, a metallophilic interaction is defined as a type of non-covalent attraction between heavy metal atoms. The atoms are often within Van der Waals distance of each other and are about as strong as hydrogen bonds. The effect can be intramolecular or intermolecular. Intermolecular metallophilic interactions can lead to formation of supramolecular assemblies whose properties vary with the choice of element and oxidation states of the metal atoms and the attachment of various ligands to them. The nature of such interactions remains the subject of vigorous debate with recent studies emphasizing that the metallophilic interaction is repulsive due to strong metal-metal Pauli exclusion principle repulsion. Nature of the interaction Previously, this type of interaction was considered to be enhanced by relativistic effects. A major contributor is electron correlation of the closed-shell components, which is unusual because closed-shell atoms generally have negligible int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Closed-shell
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is , meaning that the 1s, 2s, and 2p subshells are occupied by two, two, and six electrons, respectively. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by the nuclei and all the other electrons. Mathematically, configurations are described by Slater determinants or configuration state functions. According to the laws of quantum mechanics, a level of energy is associated with each electron configuration. In certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon. Knowledge of the electron configuration of different atoms is useful in understanding the structure o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Wire
Molecular wires (or sometimes called molecular nanowires) are molecular chains that conduct electric current. They are the proposed building blocks for molecular electronic devices. Their typical diameters are less than three nanometers, while their lengths may be macroscopic, extending to centimeters or more. Examples Most types of molecular wires are derived from organic molecules. One naturally occurring molecular wire is DNA. Prominent inorganic examples include polymeric materials such as Li2Mo6Se6 and Mo6S9−xIx, d4(CO)4(OAc)4Pd(acac)2 and single-molecule extended metal atom chains (EMACs) which comprise strings of transition metal atoms directly bonded to each other. Molecular wires containing paramagnetic inorganic moieties can exhibit Kondo peaks. Conduction of electrons Molecular wires conduct electricity. They typically have non-linear current-voltage characteristics, and do not behave as simple ohmic conductors. The conductance follows typical power law behavio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electromagnetic Spectrum
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high frequency these are: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. The electromagnetic waves in each of these bands have different characteristics, such as how they are produced, how they interact with matter, and their practical applications. Radio waves, at the low-frequency end of the spectrum, have the lowest photon energy and the longest wavelengths—thousands of kilometers, or more. They can be emitted and received by antenna (radio), antennas, and pass through the atmosphere, foliage, and most building materials. Gamma rays, at the high-frequency end of the spectrum, have the highest photon energies and the shortest wavelengths—much smaller than an atomic nucleus. Gamma rays, X-rays, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Visible Light
Light, visible light, or visible radiation is electromagnetic radiation that can be perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 terahertz. The visible band sits adjacent to the infrared (with longer wavelengths and lower frequencies) and the ultraviolet (with shorter wavelengths and higher frequencies), called collectively '' optical radiation''. In physics, the term "light" may refer more broadly to electromagnetic radiation of any wavelength, whether visible or not. In this sense, gamma rays, X-rays, microwaves and radio waves are also light. The primary properties of light are intensity, propagation direction, frequency or wavelength spectrum, and polarization. Its speed in vacuum, , is one of the fundamental constants of nature. All electromagnetic radiation exhibits some properties of both particles and waves ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
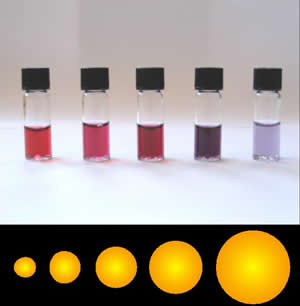
Gold Nanoparticle
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is coloured usually either wine red (for spherical particles less than 100 nm) or blue-purple (for larger spherical particles or nanorods). Due to their optical, electronic, and molecular-recognition properties, gold nanoparticles are the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine. The properties of colloidal gold nanoparticles, and thus their potential applications, depend strongly upon their size and shape. For example, rodlike particles have both a transverse and longitudinal absorption peak, and anisotropy of the shape affects their self-assembly. History Used since ancient times as a method of staining glass, colloidal gold was used in the 4th-century Lycurgus Cup, which changes color dependi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Luminescence
Luminescence is a spontaneous emission of radiation from an electronically or vibrationally excited species not in thermal equilibrium with its environment. A luminescent object emits ''cold light'' in contrast to incandescence, where an object only emits light after heating. Generally, the emission of light is due to the movement of electrons between different energy levels within an atom after excitation by external factors. However, the exact mechanism of light emission in vibrationally excited species is unknown. The dials, hands, scales, and signs of aviation and navigational instruments and markings are often coated with luminescent materials in a process known as ''luminising''. Types * Ionoluminescence, a result of bombardment by fast ions * Radioluminescence, a result of bombardment by ionizing radiation * Electroluminescence, a result of an electric current passed through a substance ** Cathodoluminescence, a result of a luminescent material being struck by elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for Chemical polarity#Polarity of molecules, polar molecules, and the most common solvent used by living things; all the ions and proteins in a Cell (biology), cell are dissolved in water within the cell. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for Organic compound, organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents (D-limonene, citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
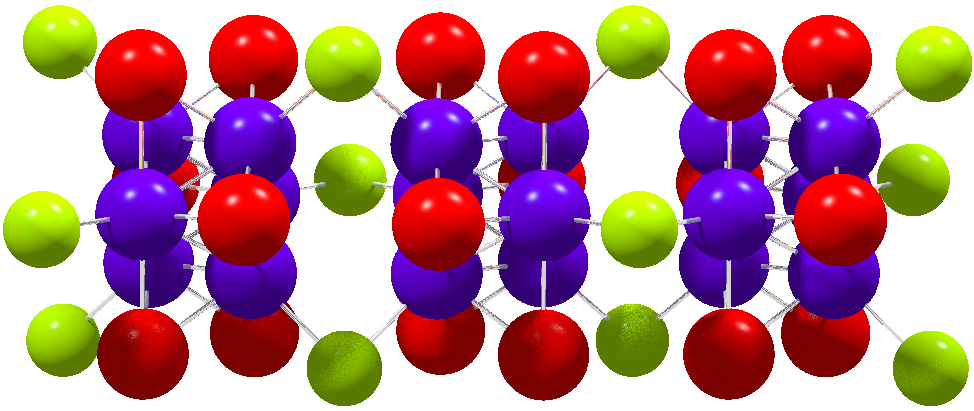
Aurophilicity
In chemistry, aurophilicity refers to the tendency of gold complexes to aggregate via formation of weak metallophilic interactions. The main evidence for aurophilicity is from the crystallographic analysis of Au(I) complexes. The aurophilic bond has a length of about 3.0 Å and a strength of about 7–12 kcal/mol, which is comparable to the strength of a hydrogen bond. The effect is greatest for gold as compared with copper or silver—the higher elements in its periodic table group—due to increased relativistic effects. Observations and theory show that, on average, 28% of the binding energy in the aurophilic interaction can be attributed to relativistic expansion of the gold d orbitals. An example of aurophilicity is the propensity of gold centres to aggregate. While both intramolecular and intermolecular aurophilic interactions have been observed, only intramolecular aggregation has been observed at such nucleation sites. Role in self-assembly The simi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is , meaning that the 1s, 2s, and 2p subshells are occupied by two, two, and six electrons, respectively. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by the nuclei and all the other electrons. Mathematically, configurations are described by Slater determinants or configuration state functions. According to the laws of quantum mechanics, a level of energy is associated with each electron configuration. In certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon. Knowledge of the electron configuration of different atoms is useful in understanding the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal, a group 11 element, and one of the noble metals. It is one of the least reactivity (chemistry), reactive chemical elements, being the second-lowest in the reactivity series. It is solid under standard temperature and pressure, standard conditions. Gold often occurs in free elemental (native state (metallurgy), native state), as gold nugget, nuggets or grains, in rock (geology), rocks, vein (geology), veins, and alluvial deposits. It occurs in a solid solution series with the native element silver (as in electrum), naturally alloyed with other metals like copper and palladium, and mineral inclusions such as within pyrite. Less commonly, it occurs in minerals as gold compounds, often with tellurium (gold tellurides). Gold is resistant to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |