|
Metallic Radius
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be described as the sharing of ''free'' electrons among a structure of positively charged ions (cations). Metallic bonding accounts for many physical properties of metals, such as strength, ductility, thermal and electrical resistivity and conductivity, opacity, and lustre. Metallic bonding is not the only type of chemical bonding a metal can exhibit, even as a pure substance. For example, elemental gallium consists of covalently-bound pairs of atoms in both liquid and solid-state—these pairs form a crystal structure with metallic bonding between them. Another example of a metal–metal covalent bond is the mercurous ion (). History As chemistry developed into a science, it became clear that metals formed the majority of the periodic table o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallic Bonding Example
Metallic may be a reference to: *Metal *Metalloid, metal-like substance *Metallic bonding, type of chemical bonding *Metallicity, in astronomy the proportion of elements other than helium and hydrogen in an object *Metallic color, a color that gives the appearance of metal *Metallic dragon, a classification of dragon found in the role playing game Dungeons & Dragons *Metallic paint, paint that provides the appearance of metal *Heavy metal music Heavy metal (or simply metal) is a Music genre, genre of rock music that developed in the late 1960s and early 1970s, largely in the United Kingdom and United States. With roots in blues rock, psychedelic rock and acid rock, heavy metal band ..., a genre of rock music See also * Metallica (other) * Metal (other) * {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodic Table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which states that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. The table is divided into four roughly rectangular areas called blocks. Elements in the same group tend to show similar chemical characteristics. Vertical, horizontal and diagonal trends characterize the periodic table. Metallic character increases going down a group and from right to left across a period. Nonmetallic character increases going from the bottom left of the periodic table to the top right. The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869; he formulated the periodic law as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have properties that differ from those of the pure elements from which they are made. The vast majority of metals used for commercial purposes are alloyed to improve their properties or behavior, such as increased strength, hardness or corrosion resistance. Metals may also be alloyed to reduce their overall cost, for instance alloys of gold and Copper(II) sulfate, copper. A typical example of an alloy is SAE 304 stainless steel, 304 grade stainless steel which is commonly used for kitchen utensils, pans, knives and forks. Sometime also known as 18/8, it as an alloy consisting broadly of 74% iron, 18% chromium and 8% nickel. The chromium and nickel alloying elements add strength and hardness to the majority iron element, but their main function is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermetallic
An intermetallic (also called intermetallic compound, intermetallic alloy, ordered intermetallic alloy, long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic elements. Intermetallics are generally hard and brittle, with good high-temperature mechanical properties. They can be classified as stoichiometric or nonstoichiometic. The term "intermetallic compounds" applied to solid phases has long been in use. However, Hume-Rothery argued that it misleads, suggesting a fixed stoichiometry and a clear decomposition into species. Definitions Research definition In 1967 defined intermetallic compounds as ''solid phases containing two or more metallic elements, with optionally one or more non-metallic elements, whose crystal structure differs from that of the other constituents''. This definition includes: * Electron (or Hume-Rothery) compounds * Size packing phases. e.g. Laves phases, Frank–Kasper phases and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Analysis
Thermal analysis is a branch of materials science where the properties of materials are studied as they change with temperature. Several methods are commonly used – these are distinguished from one another by the property which is measured: * Dielectric thermal analysis: dielectric permittivity and loss factor * Differential thermal analysis: temperature difference versus temperature or time * Differential scanning calorimetry: heat flow changes versus temperature or time * Dilatometer, Dilatometry: volume changes with temperature change * Dynamic mechanical analysis: measures storage modulus (stiffness) and loss modulus (damping) versus temperature, time and frequency * Evolved gas analysis: analysis of gases evolved during heating of a material, usually decomposition products * Isothermal titration calorimetry * Isothermal microcalorimetry * Laser flash analysis: thermal diffusivity and thermal conductivity * Thermogravimetric analysis: mass change versus temperature or time ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
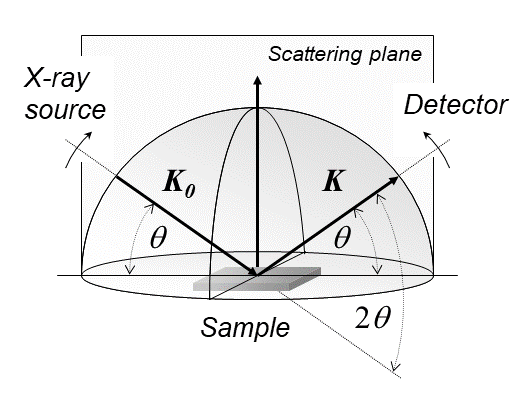
X-ray Diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. The resulting map of the directions of the X-rays far from the sample is called a diffraction pattern. It is different from X-ray crystallography which exploits X-ray diffraction to determine the arrangement of atoms in materials, and also has other components such as ways to map from experimental diffraction measurements to the positions of atoms. This article provides an overview of X-ray diffraction, starting with the early #History, history of x-rays and the discovery that they have the right spacings to be diffracted by crystals. In many cases these diffraction patterns can be #Introduction to x-ray diffraction theory, Interpreted using a single scattering or kinematical theory with conservation of energy (#Ewald's sphere, wave vecto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brillouin Zone
In mathematics and solid state physics, the first Brillouin zone (named after Léon Brillouin) is a uniquely defined primitive cell in reciprocal space Reciprocal lattice is a concept associated with solids with translational symmetry which plays a major role in many areas such as X-ray diffraction, X-ray and Electron diffraction, electron diffraction as well as the Electronic band structure, e .... In the same way the Bravais lattice is divided up into Wigner–Seitz cells in the real lattice, the reciprocal lattice is broken up into Brillouin zones. The boundaries of this cell are given by planes related to points on the reciprocal lattice. The importance of the Brillouin zone stems from the description of waves in a periodic medium given by Bloch's theorem, in which it is found that the solutions can be completely characterized by their behavior in a single Brillouin zone. The first Brillouin zone is the locus of points in reciprocal space that are closer to the or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermi Surface
In condensed matter physics, the Fermi surface is the surface in reciprocal space which separates occupied electron states from unoccupied electron states at zero temperature. The shape of the Fermi surface is derived from the periodicity and symmetry of the crystalline lattice and from the occupation of electronic band structure, electronic energy bands. The existence of a Fermi surface is a direct consequence of the Pauli exclusion principle, which allows a maximum of one electron per quantum state. The study of the Fermi surfaces of materials is called fermiology. Theory Consider a Spin (physics), spin-less ideal Fermi gas of N particles. According to Fermi–Dirac statistics, the mean occupation number of a state with energy \epsilon_i is given by : \langle n_i\rangle =\frac, where * \left\langle n_i\right\rangle is the mean occupation number of the ith state * \epsilon_i is the kinetic energy of the ith state * \mu is the chemical potential (at zero temperature, this is th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wave Vector
In physics, a wave vector (or wavevector) is a vector used in describing a wave, with a typical unit being cycle per metre. It has a magnitude and direction. Its magnitude is the wavenumber of the wave (inversely proportional to the wavelength), and its direction is perpendicular to the wavefront. In isotropic media, this is also the direction of wave propagation. A closely related vector is the angular wave vector (or angular wavevector), with a typical unit being radian per metre. The wave vector and angular wave vector are related by a fixed constant of proportionality, 2 radians per cycle. It is common in several fields of physics to refer to the angular wave vector simply as the ''wave vector'', in contrast to, for example, crystallography. It is also common to use the symbol for whichever is in use. In the context of special relativity, a '' wave four-vector'' can be defined, combining the (angular) wave vector and (angular) frequency. Definition The terms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnitude (vector)
In mathematics, a norm is a function from a real or complex vector space to the non-negative real numbers that behaves in certain ways like the distance from the origin: it commutes with scaling, obeys a form of the triangle inequality, and zero is only at the origin. In particular, the Euclidean distance in a Euclidean space is defined by a norm on the associated Euclidean vector space, called the Euclidean norm, the 2-norm, or, sometimes, the magnitude or length of the vector. This norm can be defined as the square root of the inner product of a vector with itself. A seminorm satisfies the first two properties of a norm but may be zero for vectors other than the origin. A vector space with a specified norm is called a normed vector space. In a similar manner, a vector space with a seminorm is called a ''seminormed vector space''. The term pseudonorm has been used for several related meanings. It may be a synonym of "seminorm". It can also refer to a norm that can take inf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nearly Free Electron Model
In solid-state physics, the nearly free electron model (or NFE model and quasi-free electron model) is a quantum mechanical model of physical properties of electrons that can move almost freely through the crystal lattice of a solid. The model is closely related to the more conceptual empty lattice approximation. The model enables understanding and calculation of the electronic band structures, especially of metals. This model is an immediate improvement of the free electron model, in which the metal was considered as a non-interacting electron gas and the ions were neglected completely. Mathematical formulation The nearly free electron model is a modification of the free-electron gas model which includes a ''weak'' periodic perturbation meant to model the interaction between the conduction electrons and the ions in a crystalline solid. This model, like the free-electron model, does not take into account electron–electron interactions; that is, the independent el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |