|
Metal Oxide Adhesion
The strength of metal oxide adhesion effectively determines the wetting of the metal-oxide interface. The strength of this adhesion is important, for instance, in production of light bulbs and Composite material, fiber-matrix composites that depend on the optimization of wetting to create metal-ceramic interfaces. The strength of adhesion also determines the extent of dispersion on Catalysis, catalytically active metal. Metal oxide adhesion is important for applications such as complementary metal oxide semiconductor devices. These devices make possible the high packing densities of modern integrated circuits. Oxide thermodynamics Metal oxides are formed consistent with minimizing surface energy and minimizing system entropy. The formation reactions are chemical in nature, forming bonds between oxygen dimers and pure metals or metal alloys. The reactions are endothermic for transition metals and semi-metals. At isothermic and isobaric conditions at atmosphere, the probability for a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wetting
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with the first one. The degree of wetting (wettability) is determined by a force balance between adhesive and cohesive forces. Wetting is important in the bonding or adherence of two materials. Wetting and the surface forces that control wetting are also responsible for other related effects, including capillary effects. There are two types of wetting: non-reactive wetting and reactive wetting. Wetting deals with three phases of matter: gas, liquid, and solid. It is now a center of attention in nanotechnology and nanoscience studies due to the advent of many nanomaterials in the past two decades (e.g. graphene, Carbon nano tube, carbon nanotube, boron nitride nanomesh). Explanation Adhesive forces between a liquid and solid cause a liq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
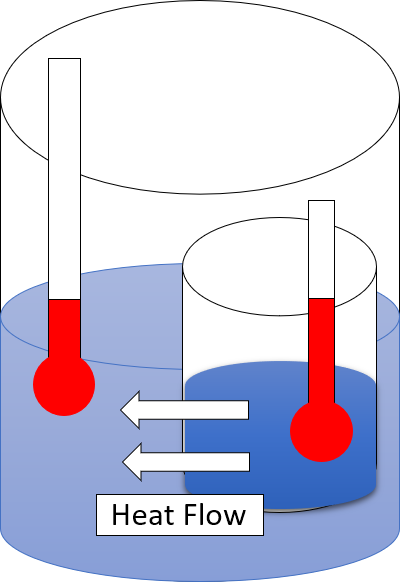
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects (or "downhill"), unless energy in some form is supplied to reverse the direction of heat flow. Another definition is: "Not all heat energy can be converted into work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics in other versions establishes the concept of entropy as a physical property of a thermodynamic system. It can be used to predict whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. The second law may be formulated by the observation that the entropy of isolated systems left to spontaneous evolution cannot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallographic Defect
A crystallographic defect is an interruption of the regular patterns of arrangement of atoms or molecules in crystalline solids. The positions and orientations of particles, which are repeating at fixed distances determined by the unit cell parameters in crystals, exhibit a periodic crystal structure, but this is usually imperfect.Ehrhart, P. (1991Properties and interactions of atomic defects in metals and alloys, volume 25 of Landolt-Börnstein, New Series III, chapter 2, p. 88, Springer, Berlin Several types of defects are often characterized: point defects, line defects, planar defects, bulk defects. Topological homotopy establishes a mathematical method of characterization. Point defects Point defects are defects that occur only at or around a single lattice point. They are not extended in space in any dimension. Strict limits for how small a point defect is are generally not defined explicitly. However, these defects typically involve at most a few extra or missing atoms. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polarizability
Polarizability usually refers to the tendency of matter, when subjected to an electric field, to acquire an electric dipole moment in proportion to that applied field. It is a property of all matter, considering that matter is made up of elementary particles which have an electric charge, namely protons and electrons. When subject to an electric field, the negatively charged electrons and positively charged atomic nuclei are subject to opposite forces and undergo charge separation. Polarizability is responsible for a material's dielectric constant and, at high (optical) frequencies, its refractive index. The polarizability of an atom or molecule is defined as the ratio of its induced dipole moment to the local electric field; in a crystalline solid, one considers the dipole moment per unit cell. Note that the local electric field seen by a molecule is generally different from the macroscopic electric field that would be measured externally. This discrepancy is taken into account by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Miller Indices
Miller indices form a notation system in crystallography for lattice planes in crystal (Bravais) lattices. In particular, a family of lattice planes of a given (direct) Bravais lattice is determined by three integers ''h'', ''k'', and ''ℓ'', the ''Miller indices''. They are written (hkℓ), and denote the family of (parallel) lattice planes (of the given Bravais lattice) orthogonal to \mathbf_ = h\mathbf + k\mathbf + \ell\mathbf, where \mathbf are the basis or primitive translation vectors of the reciprocal lattice for the given Bravais lattice. (Note that the plane is not always orthogonal to the linear combination of direct or original lattice vectors h\mathbf + k\mathbf + \ell\mathbf because the direct lattice vectors need not be mutually orthogonal.) This is based on the fact that a reciprocal lattice vector \mathbf (the vector indicating a reciprocal lattice point from the reciprocal lattice origin) is the wavevector of a plane wave in the Fourier series of a spat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bravais Lattice
In geometry and crystallography, a Bravais lattice, named after , is an infinite array of discrete points generated by a set of discrete translation operations described in three dimensional space by : \mathbf = n_1 \mathbf_1 + n_2 \mathbf_2 + n_3 \mathbf_3, where the ''ni'' are any integers, and a''i'' are ''primitive translation vectors'', or ''primitive vectors'', which lie in different directions (not necessarily mutually perpendicular) and span the lattice. The choice of primitive vectors for a given Bravais lattice is not unique. A fundamental aspect of any Bravais lattice is that, for any choice of direction, the lattice appears exactly the same from each of the discrete lattice points when looking in that chosen direction. The Bravais lattice concept is used to formally define a ''crystalline arrangement'' and its (finite) frontiers. A crystal is made up of one or more atoms, called the ''basis'' or ''motif'', at each lattice point. The ''basis'' may consist of atoms, mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charge Conservation
In physics, charge conservation is the principle that the total electric charge in an isolated system never changes. The net quantity of electric charge, the amount of positive charge minus the amount of negative charge in the universe, is always '' conserved''. Charge conservation, considered as a physical conservation law, implies that the change in the amount of electric charge in any volume of space is exactly equal to the amount of charge flowing into the volume minus the amount of charge flowing out of the volume. In essence, charge conservation is an accounting relationship between the amount of charge in a region and the flow of charge into and out of that region, given by a continuity equation between charge density \rho(\mathbf) and current density \mathbf(\mathbf). This does not mean that individual positive and negative charges cannot be created or destroyed. Electric charge is carried by subatomic particles such as electrons and protons. Charged particles ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Defect Energy
A defect is a physical, functional, or aesthetic attribute of a product or service that exhibits that the product or service failed to meet one of the desired specifications. Defect, defects or defected may also refer to: Examples * Angular defect, failure of some angles to add up to the expected amount of 360° or 180°, when such angles in the Euclidean plane would * Birth defect, a condition present at birth * Crystallographic defect, defects in the crystal lattice of solid materials *Latent defect, a fault in a property which could not have been discovered by a reasonably thorough inspection before completion or acquisition. * Product defect, a characteristic of a product which hinders its usability ** Software bug, a failure of computer software to meet requirements * Social defect, a sociological disorder Music * Defected Records, a music label * The Defects, a Northern-Irish punk rock band Other uses * Defect, the action of defection, abandoning allegiance to one country ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Roughness
Surface roughness, often shortened to roughness, is a component of surface finish (surface texture). It is quantified by the deviations in the direction of the normal vector of a real surface from its ideal form. If these deviations are large, the surface is rough; if they are small, the surface is smooth. In surface metrology, roughness is typically considered to be the high-frequency, short-wavelength component of a measured surface. However, in practice it is often necessary to know both the amplitude and frequency to ensure that a surface is fit for a purpose. Roughness plays an important role in determining how a real object will interact with its environment. In tribology, rough surfaces usually wear more quickly and have higher friction coefficients than smooth surfaces. Roughness is often a good predictor of the performance of a mechanical component, since irregularities on the surface may form nucleation sites for cracks or corrosion. On the other hand, roughness ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler substances by any chemical reaction. The number of protons in the nucleus is the defining property of an element, and is referred to as its atomic number (represented by the symbol ''Z'') – all atoms with the same atomic number are atoms of the same element. Almost all of the baryonic matter of the universe is composed of chemical elements (among rare exceptions are neutron stars). When different elements undergo chemical reactions, atoms are rearranged into new compounds held together by chemical bonds. Only a minority of elements, such as silver and gold, are found uncombined as relatively pure native element minerals. Nearly all other naturally occurring elements occur in the Earth as compounds or mixtures. Air is primarily a m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit followed by electrons around an atom's Atomic nucleus, nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (or "L shell"), then the "3 shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond to the principal quantum numbers (''n'' = 1, 2, 3, 4 ...) or are labeled alphabetically with the letters used in X-ray notation (K, L, M, ...). A useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements represents an electron shell. Each shell can contain only a fixed number of electrons: the first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on. The general formula is that the ''n''th shell can in principle hold up to 2(square ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metals
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can use d orbitals as Valence electron#Valence_shell, valence orbitals to form chemical bonds. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. Since they are metals, they are lustrous and have good electrical and thermal conductivity. Most (with the exception of Group 11 element, group 11 and Group 12 element, group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



%2C_black_and_white.png)

