|
Lyman-alpha Line
The Lyman-alpha line, typically denoted by Ly-α, is a spectral line of hydrogen (or, more generally, of any Hydrogen-like atom, one-electron atom) in the Lyman series. It is emitted when the atomic electron transitions from an ''n'' = 2 atomic orbital, orbital to the ground state (''n'' = 1), where ''n'' is the principal quantum number. In hydrogen, its wavelength of 1215.67 angstroms ( or ), corresponding to a frequency of about , places Lyman-alpha in the ultraviolet (UV) part of the electromagnetic spectrum. More specifically, Ly-α lies in vacuum ultraviolet, vacuum UV (VUV), characterized by a strong absorption in the air. Fine structure image:Hydrogen fine structure2.svg, The Lyman-alpha doublet. Because of the spin–orbit interaction, the Lyman-alpha line splits into a fine structure, fine-structure doublet with the wavelengths of 1215.668 and 1215.674 angstroms. These components are called Ly-α3/2 and Ly-α1/2, respectively. The eigenstates of the Per ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectral Line
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules. These "fingerprints" can be compared to the previously collected ones of atoms and molecules, and are thus used to identify the atomic and molecular components of stars and planets, which would otherwise be impossible. Types of line spectra Spectral lines are the result of interaction between a quantum system (usually atoms, but sometimes molecules or atomic nuclei) and a single photon. When a photon has about the right amount of energy (which is connected to its frequency) to allow a change in the energy state of the system (in the case of an atom this is usually an electron changing orbitals), the photon is absorbed. Then the energy will be spontaneously re-emitted, either as one photon at the same frequenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hamiltonian (quantum Mechanics)
Hamiltonian may refer to: * Hamiltonian mechanics, a function that represents the total energy of a system * Hamiltonian (quantum mechanics), an operator corresponding to the total energy of that system ** Dyall Hamiltonian, a modified Hamiltonian with two-electron nature ** Molecular Hamiltonian, the Hamiltonian operator representing the energy of the electrons and nuclei in a molecule * Hamiltonian (control theory), a function used to solve a problem of optimal control for a dynamical system * Hamiltonian path, a path in a graph that visits each vertex exactly once * Hamiltonian group, a non-abelian group the subgroups of which are all normal * Hamiltonian economic program, the economic policies advocated by Alexander Hamilton, the first United States Secretary of the Treasury See also * Alexander Hamilton (1755 or 1757–1804), American statesman and one of the Founding Fathers of the US * Hamilton (other) Hamilton may refer to: People * Hamilton (name), a common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emission Spectroscopy
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a transition from a high energy state to a lower energy state. The photon energy of the emitted photon is equal to the energy difference between the two states. There are many possible electron transitions for each atom, and each transition has a specific energy difference. This collection of different transitions, leading to different radiated wavelengths, make up an emission spectrum. Each element's emission spectrum is unique. Therefore, spectroscopy can be used to identify elements in matter of unknown composition. Similarly, the emission spectra of molecules can be used in chemical analysis of substances. Emission In physics, emission is the process by which a higher energy quantum mechanical state of a particle becomes converted to a lower one through the emission of a photon, resulting in the production of light. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Siegbahn Notation
The Siegbahn notation is used in X-ray spectroscopy to name the spectral lines that are characteristic to elements. It was introduced by Manne Siegbahn. The characteristic lines in X-ray emission spectra correspond to atomic electronic transitions where an electron jumps down to a vacancy in one of the inner shells of an atom. Such a hole in an inner shell may have been produced by bombardment with electrons in an X-ray tube, by other particles as in PIXE, by other X-rays in X-ray fluorescence or by radioactive decay of the atom's nucleus. Although still widely used in spectroscopy, this notation is unsystematic and often confusing. For these reasons, International Union of Pure and Applied Chemistry (IUPAC) recommends another nomenclature. History The use of the letters K and L to denote X-rays originates in a 1911 paper by Charles Glover Barkla, titled ''The Spectra of the Fluorescent Röntgen Radiations'' ("Röntgen radiation" is an archaic name for "X-rays"). By 1913, Henry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lyman-break Galaxy
Lyman-break galaxies are star-forming galaxies at high redshift that are selected using the differing appearance of the galaxy in several imaging filters due to the position of the Lyman limit. The technique has primarily been used to select galaxies at redshifts of ''z'' = 3–4 using ultraviolet and optical filters, but progress in ultraviolet astronomy and in infrared astronomy has allowed the use of this technique at lower and higher redshifts using ultraviolet and near-infrared filters. The Lyman-break galaxy selection technique relies on the fact that radiation at higher energies than the Lyman limit at 912 Å is almost completely absorbed by neutral gas around star-forming regions of galaxies. In the rest frame of the emitting galaxy, the emitted spectrum is bright at wavelengths longer than 912 Å, but very dim or imperceptible at shorter wavelengths—this is known as a " dropout", or "break", and can be used to find the position of the Lyman limit. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lyman-alpha Blob
In astronomy, a Lyman-alpha blob (LAB) is a huge concentration of a gas emitting the Lyman-alpha emission line. LABs are some of the largest known individual objects in the Universe. Some of these gaseous structures are more than 400,000 light years across. So far they have only been found in the high-redshift universe because of the ultraviolet nature of the Lyman-alpha emission line. Since Earth's atmosphere is very effective at filtering out UV photons, the Lyman-alpha photons must be redshifted in order to be transmitted through the atmosphere. The most famous Lyman-alpha blobs were discovered in 2000 by Steidel et al. Matsuda et al., using the Subaru Telescope of the National Astronomical Observatory of Japan extended the search for LABs and found over 30 new LABs in the original field of Steidel et al., although they were all smaller than the originals. These LABs form a structure which is more than 200 million light-years in extent. It is currently unknown whether LABs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lyman-alpha Emitter
A Lyman-alpha emitter (LAE) is a type of distant galaxy that emits Lyman-alpha radiation from neutral hydrogen. Most known LAEs are extremely distant, and because of the finite travel time of light they provide glimpses into the history of the universe. They are thought to be the progenitors of most modern Milky Way type galaxies. These galaxies can be found nowadays rather easily in narrow-band searches by an excess of their narrow-band flux at a wavelength which may be interpreted from their redshift: : 1+z=\frac where z is the redshift, \lambda is the observed wavelength, and 1215.67 Å is the wavelength of Lyman-alpha emission. The Lyman-alpha line in most LAEs is thought to be caused by recombination of interstellar hydrogen that is ionized by an ongoing burst of star-formation. Such Lyman alpha emission was first suggested as a signature of young galaxies by Bruce Partridge and P. J. E. Peebles in 1967. Experimental observations of the redshift of LAEs are important in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lyman-alpha Forest
The Lyman-alpha line, typically denoted by Ly-α, is a spectral line of hydrogen (or, more generally, of any one-electron atom) in the Lyman series. It is emitted when the atomic electron transitions from an ''n'' = 2 orbital to the ground state (''n'' = 1), where ''n'' is the principal quantum number. In hydrogen, its wavelength of 1215.67 angstroms ( or ), corresponding to a frequency of about , places Lyman-alpha in the ultraviolet (UV) part of the electromagnetic spectrum. More specifically, Ly-α lies in vacuum UV (VUV), characterized by a strong absorption in the air. Fine structure The Lyman-alpha doublet. Because of the spin–orbit interaction, the Lyman-alpha line splits into a fine-structure doublet with the wavelengths of 1215.668 and 1215.674 angstroms. These components are called Ly-α3/2 and Ly-α1/2, respectively. The eigenstates of the perturbed Hamiltonian are labeled by the ''total'' angular momentum ''j'' of the electron, not just the o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Electrodynamics
In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction. In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it "the jewel of physics" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen. History The first formulation of a quantum theory describing radiation and matter interaction is attributed to British scientist Paul Dirac, who ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
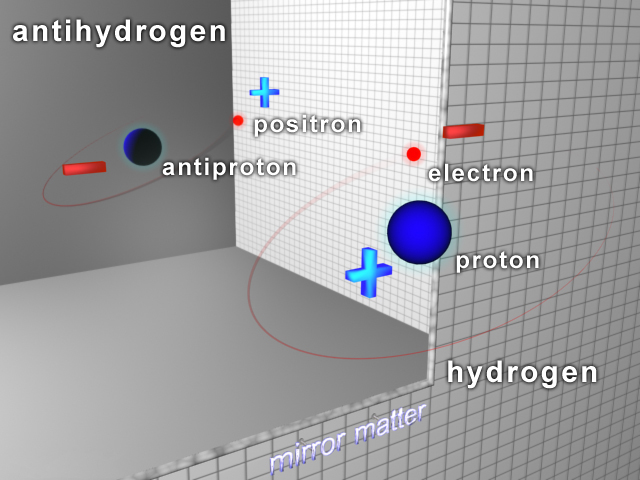
Antihydrogen
Antihydrogen () is the antimatter counterpart of hydrogen. Whereas the common hydrogen atom is composed of an electron and proton, the antihydrogen atom is made up of a positron and antiproton. Scientists hope that studying antihydrogen may shed light on the question of why there is more matter than antimatter in the observable universe, known as the baryon asymmetry problem. Antihydrogen is produced artificially in particle accelerators. Experimental history Accelerators first detected hot antihydrogen in the 1990s. ATHENA studied cold in 2002. It was first trapped by the Antihydrogen Laser Physics Apparatus (ALPHA Collaboration, ALPHA) team at CERN in 2010, who then measured the structure and other important properties. ALPHA, AEGIS, and GBAR plan to further cool and study atoms. 1s–2s transition measurement In 2016, the ALPHA experiment measured the atomic electron transition between the two lowest energy levels of antihydrogen, 1s–2s. The results, which are identical t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's surface is made up of the ocean, dwarfing Earth's polar ice, lakes, and rivers. The remaining 29% of Earth's surface is land, consisting of continents and islands. Earth's surface layer is formed of several slowly moving tectonic plates, which interact to produce mountain ranges, volcanoes, and earthquakes. Earth's liquid outer core generates the magnetic field that shapes the magnetosphere of the Earth, deflecting destructive solar winds. The atmosphere of the Earth consists mostly of nitrogen and oxygen. Greenhouse gases in the atmosphere like carbon dioxide (CO2) trap a part of the energy from the Sun close to the surface. Water vapor is widely present in the atmosphere and forms clouds that cover most of the planet. More solar e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redshift
In physics, a redshift is an increase in the wavelength, and corresponding decrease in the frequency and photon energy, of electromagnetic radiation (such as light). The opposite change, a decrease in wavelength and simultaneous increase in frequency and energy, is known as a negative redshift, or blueshift. The terms derive from the colours red and blue which form the extremes of the visible light spectrum. In astronomy and cosmology, the three main causes of electromagnetic redshift are # The radiation travels between objects which are moving apart (" relativistic" redshift, an example of the relativistic Doppler effect) #The radiation travels towards an object in a weaker gravitational potential, i.e. towards an object in less strongly curved (flatter) spacetime (gravitational redshift) #The radiation travels through expanding space (cosmological redshift). The observation that all sufficiently distant light sources show redshift corresponding to their distance from Earth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |