|
List Of Hund's Rules
In atomic physics, Hund's rules refers to a set of rules that German physicist Friedrich Hund formulated around 1927, which are used to determine the term symbol that corresponds to the ground state of a multi-electron atom. The first rule is especially important in chemistry, where it is often referred to simply as Hund's Rule. The three rules are: # For a given electron configuration, the term with maximum multiplicity has the lowest energy. The multiplicity is equal to 2S + 1 \ , where S is the total spin angular momentum for all electrons. The multiplicity is also equal to the number of unpaired electrons plus one.Miessler and Tarr p.33 Therefore, the term with lowest energy is also the term with maximum S \, and maximum number of unpaired electrons. # For a given multiplicity, the term with the largest value of the total orbital angular momentum quantum number L \, has the lowest energy. # For a given term, in an atom with outermost subshell half-filled or less, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Physics
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. Atomic physics typically refers to the study of atomic structure and the interaction between atoms. It is primarily concerned with the way in which electrons are arranged around the nucleus and the processes by which these arrangements change. This comprises ions, neutral atoms and, unless otherwise stated, it can be assumed that the term ''atom'' includes ions. The term ''atomic physics'' can be associated with nuclear power and nuclear weapons, due to the synonymous use of ''atomic'' and ''nuclear'' in standard English. Physicists distinguish between atomic physics—which deals with the atom as a system consisting of a nucleus and electrons—and nuclear physics, which studies nuclear reactions and special properties of atomic nuclei. As with many scientific fields, strict delineation can be highly contrived and atomic physics is often considered in the wider c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Effective Nuclear Charge
In atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an electron in a multi-electron atom. The term "effective" is used because the shielding effect of negatively charged electrons prevent higher energy electrons from experiencing the full nuclear charge of the nucleus due to the repelling effect of inner layer. The effective nuclear charge experienced by an electron is also called the core charge. It is possible to determine the strength of the nuclear charge by the oxidation number of the atom. Most of the physical and chemical properties of the elements can be explained on the basis of electronic configuration. Consider the behavior of ionization energies in the periodic table. It is known that the magnitude of ionization potential depends upon the following factors: # Size of atom; # The nuclear charge; # The screening effect of the inner shells, and # The extent to which the outermost electron penetrates into the charge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photochemistry
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400–750 nm) or infrared radiation (750–2500 nm). In nature, photochemistry is of immense importance as it is the basis of photosynthesis, vision, and the formation of vitamin D with sunlight. Photochemical reactions proceed differently than temperature-driven reactions. Photochemical paths access high energy intermediates that cannot be generated thermally, thereby overcoming large activation barriers in a short period of time, and allowing reactions otherwise inaccessible by thermal processes. Photochemistry can also be destructive, as illustrated by the photodegradation of plastics. Concept Grotthuss–Draper law and Stark-Einstein law Photoexcitation is the first step in a photochemical process where the reactant is elevated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth. It has a concentration in the Earth's crust of about one gram per kilogram (compare copper at about 0.06 grams). In minerals, phosphorus generally occurs as phosphate. Elemental phosphorus was first isolated as white phosphorus in 1669. White phosphorus emits a faint glow when exposed to oxygen – hence the name, taken from Greek mythology, meaning 'light-bearer' (Latin ), referring to the " Morning Star", the planet Venus. The term '' phosphorescence'', meaning glow after illumination, derives from this property of phosphorus, although the word has since been used for a different physical process that produces a glow. The glow of phosphorus is caused by oxidation of the white (but not red) phosphorus — a process now called chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere The greatest commercial use of the element is the production o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Good Quantum Number
In quantum mechanics, given a particular Hamiltonian H and an operator O with corresponding eigenvalues and eigenvectors given by O, q_j\rangle=q_j, q_j\rangle, the q_j are said to be good quantum numbers if every eigenvector , q_j\rangle remains an eigenvector of O ''with the same eigenvalue'' as time evolves. In other words, the eigenvalues q_j are good quantum numbers if the corresponding operator O is a constant of motion. Good quantum numbers are often used to label initial and final states in experiments. For example, in particle colliders: 1. Particles are initially prepared in approximate momentum eigenstates; the particle momentum being a good quantum number for non-interacting particles. 2. The particles are made to collide. At this point, the momentum of each particle is undergoing change and thus the particles’ momenta are not a good quantum number for the interacting particles during the collision. 3. A significant time after the collision, particles are measure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angular Momentum Operator
In quantum mechanics, the angular momentum operator is one of several related operators analogous to classical angular momentum. The angular momentum operator plays a central role in the theory of atomic and molecular physics and other quantum problems involving rotational symmetry. Such an operator is applied to a mathematical representation of the physical state of a system and yields an angular momentum value if the state has a definite value for it. In both classical and quantum mechanical systems, angular momentum (together with linear momentum and energy) is one of the three fundamental properties of motion.Introductory Quantum Mechanics, Richard L. Liboff, 2nd Edition, There are several angular momentum operators: total angular momentum (usually denoted J), orbital angular momentum (usually denoted L), and spin angular momentum (spin for short, usually denoted S). The term ''angular momentum operator'' can (confusingly) refer to either the total or the orbital angular momen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. In an ordinary uncharged atom, the atomic number is also equal to the number of electrons. For an ordinary atom, the sum of the atomic number ''Z'' and the neutron number ''N'' gives the atom's atomic mass number ''A''. Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes) and the mass defect of the nucleon binding is always small compared to the nucleon mass, the atomic mass of any atom, when expressed in unified atomic mass units (making a quantity called the "relative isotopic mass"), is within 1% of the whole number ''A''. Atoms with the same atomic number but dif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
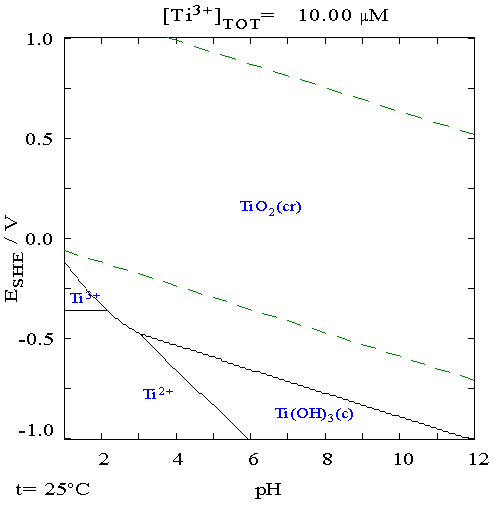
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine. Titanium was discovered in Cornwall, Great Britain, by William Gregor in 1791 and was named by Martin Heinrich Klaproth after the Titans of Greek mythology. The element occurs within a number of minerals, principally rutile and ilmenite, which are widely distributed in the Earth's crust and lithosphere; it is found in almost all living things, as well as bodies of water, rocks, and soils. The metal is extracted from its principal mineral ores by the Kroll and Hunter processes. The most common compound, titanium dioxide, is a popular photocatalyst and is used in the manufacture of white pigments. Other compounds include titanium tetrachloride (TiCl4), a component of smoke screens and catalysts; and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triplet State
In quantum mechanics, a triplet is a quantum state of a system with a spin of quantum number =1, such that there are three allowed values of the spin component, = −1, 0, and +1. Spin, in the context of quantum mechanics, is not a mechanical rotation but a more abstract concept that characterizes a particle's intrinsic angular momentum. It is particularly important for systems at atomic length scales, such as individual atoms, protons, or electrons. Almost all molecules encountered in daily life exist in a singlet state, but molecular oxygen is an exception. At room temperature, O2 exists in a triplet state, which can only undergo a chemical reaction by making the forbidden transition into a singlet state. This makes it kinetically nonreactive despite being thermodynamically one of the strongest oxidants. Photochemical or thermal activation can bring it into the singlet state, which makes it kinetically as well as thermodynamically a very strong oxidant. __TOC__ Two spin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectroscopic Notation
Spectroscopic notation provides a way to specify atomic ionization states, atomic orbitals, and molecular orbitals. Ionization states Spectroscopists customarily refer to the spectrum arising from a given ionization state of a given element by the element's symbol followed by a Roman numeral. The numeral I is used for spectral lines associated with the neutral element, II for those from the first ionization state, III for those from the second ionization state, and so on. For example, "He I" denotes lines of neutral helium, and "C IV" denotes lines arising from the third ionization state, C3+, of carbon. This notation is used for example to retrieve data from thNIST Atomic Spectrum Database Atomic and molecular orbitals Before atomic orbitals were understood, spectroscopists discovered various distinctive series of spectral lines in atomic spectra, which they identified by letters. These letters were later associated with the azimuthal quantum number, ''ℓ''. The lett ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |