|
Linear Sweep Voltammetry
In analytical chemistry, linear sweep voltammetry is a method of voltammetry where the current at a working electrode is measured while the potential between the working electrode and a reference electrode is swept linearly in time. Oxidation or reduction of species is registered as a peak or trough in the current signal at the potential at which the species begins to be oxidized or reduced. Experimental method The experimental setup for linear sweep voltammetry utilizes a potentiostat and a three-electrode setup to deliver a potential to a solution and monitor its change in current. The three-electrode setup consists of a working electrode, an auxiliary electrode, and a reference electrode. The potentiostat delivers the potentials through the three-electrode setup. A potential, , is delivered through the working electrode. The slope of the potential vs. time graph is called the ''scan rate'' and can range from mV/s to 1,000,000 V/s. The working electrode is one of the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linear Potential Sweep
In mathematics, the term ''linear'' is used in two distinct senses for two different properties: * linearity of a ''function'' (or '' mapping''); * linearity of a ''polynomial''. An example of a linear function is the function defined by f(x)=(ax,bx) that maps the real line to a line in the Euclidean plane R2 that passes through the origin. An example of a linear polynomial in the variables X, Y and Z is aX+bY+cZ+d. Linearity of a mapping is closely related to '' proportionality''. Examples in physics include the linear relationship of voltage and current in an electrical conductor (Ohm's law), and the relationship of mass and weight. By contrast, more complicated relationships, such as between velocity and kinetic energy, are ''nonlinear''. Generalized for functions in more than one dimension, linearity means the property of a function of being compatible with addition and scaling, also known as the superposition principle. Linearity of a polynomial means that its degree is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up quark, up and down quark, down quarks. Electrons are extremely lightweight particles that orbit the positively charged atomic nucleus, nucleus of atoms. Their negative charge is balanced by the positive charge of protons in the nucleus, giving atoms their overall electric charge#Charge neutrality, neutral charge. Ordinary matter is composed of atoms, each consisting of a positively charged nucleus surrounded by a number of orbiting electrons equal to the number of protons. The configuration and energy levels of these orbiting electrons determine the chemical properties of an atom. Electrons are bound to the nucleus to different degrees. The outermost or valence electron, valence electrons are the least tightly bound and are responsible for th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Voltammetry
In electrochemistry, cyclic voltammetry (CV) is a type of voltammetric measurement where the potential of the working electrode is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles in potential are repeated until the voltammetric trace reaches a cyclic steady state. The current at the working electrode is plotted versus the voltage at the working electrode to yield the cyclic voltammogram (see Figure 1). Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode. Experimental method In cyclic voltammetry (CV), the electrode potential is ramped linearly versus time in cyclical phases. The rate of voltage change over time during each of these phases is known as the scan rate (V/s). In a standar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voltammetry
Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied. The analytical data for a voltammetric experiment comes in the form of a voltammogram, which plots the current produced by the analyte versus the potential of the working electrode. Theory Voltammetry is the study of current as a function of applied potential. Voltammetric methods involve electrochemical cells, and investigate the reactions occurring at electrode/electrolyte interfaces. The reactivity of analytes in these half-cells is used to determine their concentration. It is considered a dynamic electrochemical method as the applied potential is varied over time and the corresponding changes in current are measured. Most experiments control the potential (volts) of an electrode in contact with the analyte while measuring the resulting current (am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Voltammetry
In electrochemistry, cyclic voltammetry (CV) is a type of voltammetric measurement where the potential of the working electrode is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles in potential are repeated until the voltammetric trace reaches a cyclic steady state. The current at the working electrode is plotted versus the voltage at the working electrode to yield the cyclic voltammogram (see Figure 1). Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode. Experimental method In cyclic voltammetry (CV), the electrode potential is ramped linearly versus time in cyclical phases. The rate of voltage change over time during each of these phases is known as the scan rate (V/s). In a standar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reduction Potential
Redox potential (also known as oxidation / reduction potential, ''ORP'', ''pe'', ''E_'', or E_) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respectively. Redox potential is expressed in volts (V). Each species has its own intrinsic redox potential; for example, the more positive the reduction potential (reduction potential is more often used due to general formalism in electrochemistry), the greater the species' affinity for electrons and tendency to be reduced. Measurement and interpretation In aqueous solutions, redox potential is a measure of the tendency of the solution to either gain or lose electrons in a reaction. A solution with a higher (more positive) reduction potential than some other molecule will have a tendency to gain electrons from this molecule (i.e. to be reduced by oxidizing this other molecule) and a solution with a lower (more negative) reduction potential w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffuse
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, as in spinodal decomposition. Diffusion is a stochastic process due to the inherent randomness of the diffusing entity and can be used to model many real-life stochastic scenarios. Therefore, diffusion and the corresponding mathematical models are used in several fields beyond physics, such as statistics, probability theory, information theory, neural networks, finance, and marketing. The concept of diffusion is widely used in many fields, including physics (Molecular diffusion, particle diffusion), chemistry, biology, sociology, economics, statistics, data science, and finance (diffusion of people, ideas, data and price v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solvent like water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also functi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Transfer
Electron transfer (ET) occurs when an electron relocates from an atom, ion, or molecule, to another such chemical entity. ET describes the mechanism by which electrons are transferred in redox reactions. Electrochemical processes are ET reactions. ET reactions are relevant to photosynthesis and respiration and commonly involve transition metal complexes. In organic chemistry ET is a step in some industrial polymerization reactions. It is foundational to photoredox catalysis. Classes of electron transfer Inner-sphere electron transfer In inner-sphere ET, two redox centers are covalently linked during the ET. This bridge can be permanent, in which case the electron transfer event is termed intramolecular electron transfer. More commonly, however, the covalent linkage is transitory, forming just prior to the ET and then disconnecting following the ET event. In such cases, the electron transfer is termed intermolecular electron transfer. A famous example of an inner sphere ET pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Auxiliary Electrode
Auxiliary may refer to: In language * Auxiliary language (other) * Auxiliary verb In military and law enforcement * Auxiliary police * Auxiliaries, civilians or quasi-military personnel who provide support of some kind to a military service ** Auxiliaries (Roman military) In religion * Auxiliary bishop, in the Roman Catholic Church * Auxiliary organization (LDS Church) In technology * Auxiliary input jack and auxiliary cable, generally for audio ** frequently associated with mobile device audio * Aux-send of a mixing console * An auxiliary port is a common port found on many Cisco routers for CLI access. * A backup site or system Other uses * Auxiliary route, also known as "special route", in road transportation ** An auxiliary route of the Interstate Highway System in the United States * Auxiliary ship is a naval vessel designed to operate in support of combat ships and other naval operations * Auxiliary (fraternity or sorority) * A marching band color guard * Auxil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical Chemistry
Analytical skill, Analytical chemistry studies and uses instruments and methods to Separation process, separate, identify, and Quantification (science), quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative inorganic analysis, Qualitative analysis identifies analytes, while Quantitative analysis (chemistry), quantitative analysis determines the numerical amount or concentration. Analytical chemistry consists of classical, wet chemistry, wet chemical methods and modern analytical techniques. Classical qualitative methods use separations such as Precipitation (chemistry), precipitation, Extraction (chemistry), extraction, and distillation. Identification may be based on differences in color, odor, melting point, boiling point, solubility, radioactivity or reactivity. Classical quantitative analysis uses mass or volume changes to quantify amount. Ins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
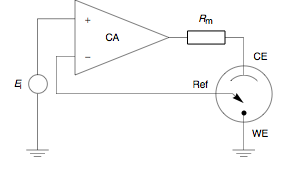
Potentiostat
A potentiostat is the electronic hardware required to control a three electrode cell and run most electroanalytical experiments. A ''Bipotentiostat'' and ''polypotentiostat'' are potentiostats capable of controlling two working electrodes and more than two working electrodes, respectively. The system functions by maintaining the potential of the working electrode at a constant level with respect to the reference electrode by adjusting the current at an auxiliary electrode. The heart of the different potentiostatic electronic circuits is an operational amplifier (op amp). It consists of an electric circuit which is usually described in terms of simple op amps. Primary use This equipment is fundamental to modern electrochemical studies using three electrode systems for investigations of reaction mechanisms related to redox chemistry and other chemical phenomena. The dimensions of the resulting data depend on the experiment. In voltammetry, electric current in amps is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |