|
Landau–de Gennes Theory
In physics, Landau–de Gennes theory describes the NI transition, i.e., phase transition between nematic liquid crystals and isotropic liquids, which is based on the classical Landau's theory and was developed by Pierre-Gilles de Gennes in 1969. The phenomonological theory uses the \mathbf tensor as an order parameter in expanding the free energy density. Mathematical description The NI transition is a first-order phase transition, albeit it is very weak. The order parameter is the \mathbf tensor, which is symmetric, traceless, second-order tensor and vanishes in the isotropic liquid phase. We shall consider a uniaxial \mathbf Q tensor, which is defined by :\mathbf Q = S(\mathbf n\otimes\mathbf n - \tfrac\mathbf I) where S=S(T) is the scalar order parameter and \mathbf n is the director. The \mathbf Q tensor is zero in the isotropic liquid phase since the scalar order parameter S is zero, but becomes non-zero in the nematic phase. Near the NI transition, the (Helmholtz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
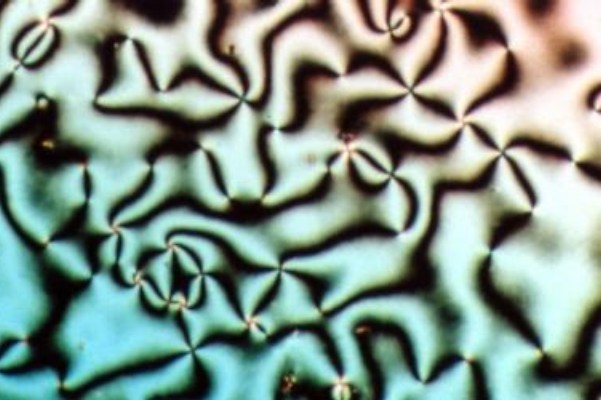
Liquid Crystals
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in a solid. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter (just as water may be ice or water vapour). Liquid crystals can be divided into three main types: thermotropic, lyotropic, and metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Landau Theory
Landau theory (also known as Ginzburg–Landau theory, despite the confusing name) in physics is a theory that Lev Landau introduced in an attempt to formulate a general theory of continuous (i.e., second-order) phase transitions. It can also be adapted to systems under externally-applied fields, and used as a quantitative model for discontinuous (i.e., first-order) transitions. Although the theory has now been superseded by the renormalization group and scaling theory formulations, it remains an exceptionally broad and powerful framework for phase transitions, and the associated concept of the Phase transitions#Order parameters, order parameter as a descriptor of the essential character of the transition has proven transformative. Mean-field formulation (no long-range correlation) Landau was motivated to suggest that the free energy of any system should obey two conditions: *Be analytic in the order parameter and its gradients. *Obey the symmetry of the Hamiltonian mechanics, H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pierre-Gilles De Gennes
Pierre-Gilles de Gennes (; 24 October 1932 – 18 May 2007) was a French physicist and the Nobel Prize laureate in physics in 1991. Education and early life He was born in Paris, France, and was home-schooled to the age of 12. By the age of 13, he had adopted adult reading habits and was visiting museums. Later, de Gennes studied at the École Normale Supérieure. After leaving the ''École'' in 1955, he became a research engineer at the Saclay center of the Commissariat à l'Énergie Atomique, working mainly on neutron scattering and magnetism, with advice from Anatole Abragam and Jacques Friedel. He defended his Ph.D. in 1957 at the University of Paris. Career and research In 1959, he was a postdoctoral research visitor with Charles Kittel at the University of California, Berkeley, and then spent 27 months in the French Navy. In 1961, he was assistant professor in Orsay and soon started the Orsay group on superconductors. In 1968, he switched to studying liquid crysta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Q Tensor
In physics, \mathbf Q-tensor is an orientational order parameter that describes uniaxial and biaxial nematic liquid crystals and vanishes in the isotropic liquid phase. The \mathbf Q tensor is a second-order, traceless, symmetric tensor and is defined byDe Gennes, P. G., & Prost, J. (1993). The physics of liquid crystals (No. 83). Oxford university press.Kleman, M., & Lavrentovich, O. D. (Eds.). (2003). Soft matter physics: an introduction. New York, NY: Springer New York. :\mathbf = S\left(\mathbf n\otimes\mathbf n - \tfrac\mathbf I\right) + R\left(\mathbf m\otimes\mathbf m - \tfrac\mathbf I\right) where S=S(T) and R=R(T) are scalar order parameters, (\mathbf n,\mathbf m) are the two directors of the nematic phase and T is the temperature; in uniaxial liquid crystals, R=0. The components of the tensor are :Q_ = S\left(n_in_j - \tfrac\delta_\right) + R\left(m_im_j - \tfrac\delta_\right) The states with directors \mathbf n and -\mathbf n are physically equivalent and similarly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Order Parameter
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition States of matter Phase transitions commonly refer to when a substance transforms between one of the four states of matter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helmholtz Free Energy
In thermodynamics, the Helmholtz free energy (or Helmholtz energy) is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature ( isothermal). The change in the Helmholtz energy during a process is equal to the maximum amount of work that the system can perform in a thermodynamic process in which temperature is held constant. At constant temperature, the Helmholtz free energy is minimized at equilibrium. In contrast, the Gibbs free energy or free enthalpy is most commonly used as a measure of thermodynamic potential (especially in chemistry) when it is convenient for applications that occur at constant ''pressure''. For example, in explosives research Helmholtz free energy is often used, since explosive reactions by their nature induce pressure changes. It is also frequently used to define fundamental equations of state of pure substances. The concept of free energy was developed by Hermann von Helmholtz, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs Free Energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–volume work, pressure–volume work, that may be performed by a closed system, thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as chemical reactions that may occur under these conditions. The Gibbs free energy is expressed as G(p,T) = U + pV - TS = H - TS where: * U is the internal energy of the system * H is the enthalpy of the system * S is the entropy of the system * T is the temperature of the system * V is the volume of the system * p is the pressure of the system (which must be equal to that of the surroundings for mechanical equilibrium). The Gibbs free energy change (, measured in joules in International System of Units, SI) is the ''maximum'' amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soft Matter
Soft matter or soft condensed matter is a type of matter that can be deformed or structurally altered by thermal or mechanical stress which is of similar magnitude to thermal fluctuations. The science of soft matter is a subfield of condensed matter physics. Soft materials include liquids, colloids, polymers, foams, gels, granular materials, liquid crystals, flesh, and a number of biomaterials. These materials share an important common feature in that predominant physical behaviors occur at an energy scale comparable with room temperature thermal energy (of order of kT), and that entropy is considered the dominant factor. At these temperatures, quantum aspects are generally unimportant. When soft materials interact favorably with surfaces, they become squashed without an external compressive force. Pierre-Gilles de Gennes, who has been called the "founding father of soft matter," received the Nobel Prize in Physics in 1991 for discovering that methods developed f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transitions
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic State of matter, states of matter: solid, liquid, and gas, and in rare cases, plasma (physics), plasma. A phase of a thermodynamic system and the states of matter have uniform physical property, physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition States of matter Phase transitions commonly refer to when a substance tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |