|
Iodopropynyl Butylcarbamate
Iodopropynyl Butyl Carbamate (IPBC) is a water-soluble preservative used globally in the paints & coatings, wood preservatives, personal care, and cosmetics industries. IPBC is a member of the carbamate family of biocides. IPBC was invented in the 1970s and has a long history of effective use as an antifungal technology. History IPBC was initially developed for use in the paint & coatings industry as a dry-film preservative to protect interior and exterior coatings from mold, mildew, and fungal growth, while also offering cost performance and sustainability benefits. IPBC exhibits efficacy against a broad spectrum of fungal species, typically at very low use levels. IPBC today is incorporated into a wide variety of interior and exterior paint formulations around the world. Use is restricted in some countries due to its toxicity, especially acute inhalation toxicity. IPBC is also becoming recognized as a contact allergen. Uses IPBC is an effective fungicide at very low concentra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
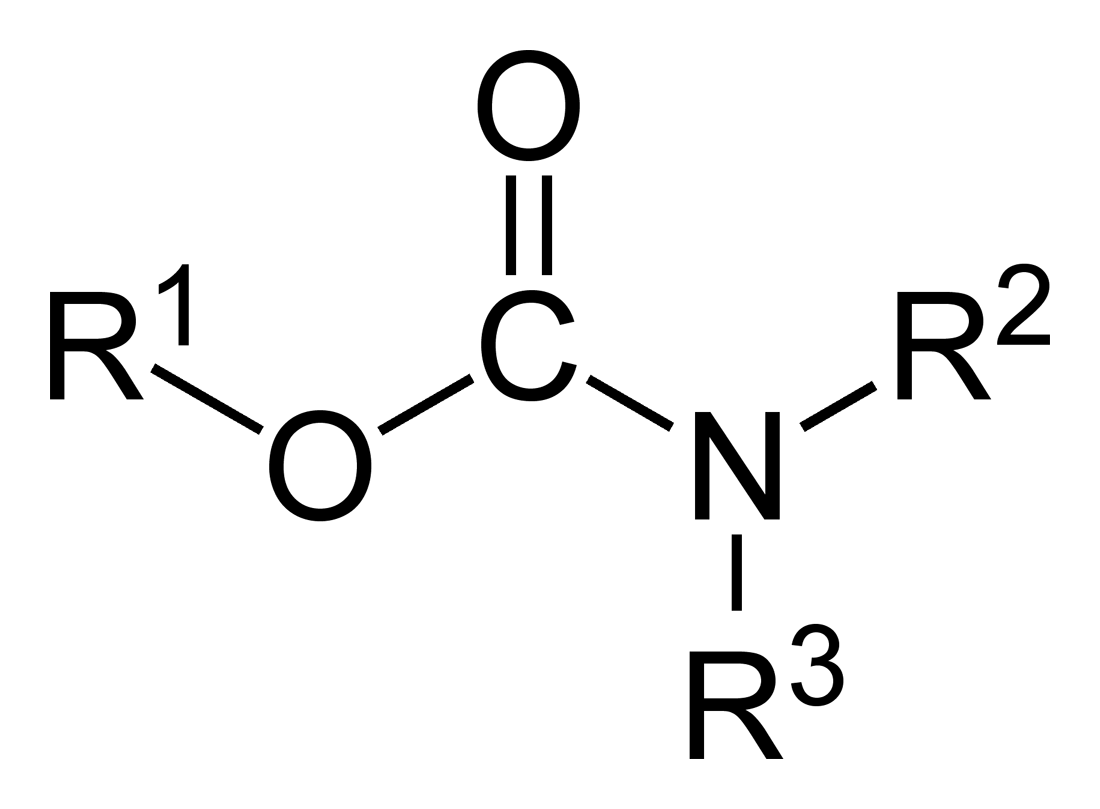
Carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose units are joined by carbamate groups are an important family of plastics, the polyurethanes. Properties While carbamic acids are unstable, many carbamate esters or ionic) are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to an ammonium carbamate/carbonate solution will precipitate some calcium carbonate immediatel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allergen
An allergen is a type of antigen that produces an abnormally vigorous immune response in which the immune system fights off a perceived threat that would otherwise be harmless to the body. Such reactions are called allergies. In technical terms, an allergen is an antigen that is capable of stimulating a type-I hypersensitivity reaction in atopic individuals through immunoglobulin E (IgE) responses. Most humans mount significant Immunoglobulin E responses only as a defense against parasitic infections. However, some individuals may respond to many common environmental antigens. This hereditary predisposition is called atopy. In atopic individuals, non-parasitic antigens stimulate inappropriate IgE production, leading to type I hypersensitivity. Sensitivities vary widely from one person (or from one animal) to another. A very broad range of substances can be allergens to sensitive individuals. Types of allergens Allergens can be found in a variety of sources, such as dust mite e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ingredients Of Cosmetics
Cosmetics ingredients come from a variety of sources but, unlike the ingredients of food, are often not considered by most consumers. Cosmetics often use vibrant colors that are derived from a wide variety of sources, ranging from crushed insects to rust.Schneider, Günther ''et al'' (2005). "Skin Cosmetics" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. Cosmetics in a variety of forms date back to early civilizations, with the need to improve ones personal appearance being an important factor in attracting a mate. Over the years the ingredients have changed dramatically as we discovered how to manufacture our own scents and cosmetic formulas. The realization of the dangers of many common ingredients also greatly affected the growing industry. Ancient Egyptian aristocracy made use of minerals to provide colour and definition to their facial features. During the era of the Greek Empire it was common to use face paints, while cosmetics in ancien ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbamates
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose units are joined by carbamate groups are an important family of plastics, the polyurethanes. Properties While carbamic acids are unstable, many carbamate esters or ionic) are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to an ammonium carbamate/carbonate solution will precipitate some calcium carbonate immediately, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Preservatives
A preservative is a substance or a chemical that is added to products such as food products, beverages, pharmaceutical drugs, paints, biological samples, cosmetics, wood, and many other products to prevent decomposition by microbial growth or by undesirable chemical changes. In general, preservation is implemented in two modes, chemical and physical. Chemical preservation entails adding chemical compounds to the product. Physical preservation entails processes such as refrigeration or drying.Erich Lück and Gert-Wolfhard von Rymon Lipinski "Foods, 3. Food Additives" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2002, Wiley-VCH, Weinheim. Preservative food additives reduce the risk of foodborne infections, decrease microbial spoilage, and preserve fresh attributes and nutritional quality. Some physical techniques for food preservation include dehydration, UV-C radiation, freeze-drying, and refrigeration. Chemical preservation and physical preservation techniques are some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cosmetics Chemicals
Cosmetics are constituted mixtures of chemical compounds derived from either natural sources, or synthetically created ones. Cosmetics have various purposes. Those designed for personal care and skin care can be used to cleanse or protect the body or skin. Cosmetics designed to enhance or alter one's appearance (makeup) can be used to conceal blemishes, enhance one's natural features (such as the eyebrows and eyelashes), add color to a person's face, or change the appearance of the face entirely to resemble a different person, creature or object. Cosmetics can also be designed to add fragrance to the body. Definition and etymology The word ''cosmetics'' derives from the Greek (), meaning "technique of dress and ornament", from (), "skilled in ordering or arranging" and that from (), meaning "order" and "ornament". Cosmetics are constituted from a mixture of chemical compounds derived from either natural sources, or synthetically created ones. Legal definition Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoiodides
Organoiodine compounds are organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt. Structure, bonding, general properties Almost all organoiodine compounds feature iodide connected to one carbon center. These are usually classified as derivatives of I−. Some organoiodine compounds feature iodine in higher oxidation states. The C–I bond is the weakest of the carbon–halogen bonds. These bond strengths correlate with the electronegativity of the halogen, decreasing in the order F > Cl > Br > I. This periodic order also follows the atomic radius of halogens and the length of the carbon-halogen bond. For example, in the molecules represented by CH3X, where X is a halide, the carbon-X bonds have strengths, or bond dissociation energies, of 115, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |