|
Industrial Catalysts
The first time a catalyst was used in the industry was in 1746 by J. Hughes in the manufacture of lead chamber sulfuric acid. Since then catalysts have been in use in a large portion of the chemical industry. In the start only pure components were used as catalysts, but after the year 1900 multicomponent catalysts were studied and are now commonly used in the industry.Jacobs, G., Davis, B. H.,(2007) Low temperature water-gas shift catalysts, In ''Catalysis'', vol 20, Spivey, J.J and Dooley, K.M (Ed), Cambridge, The royal society of chemistry In the chemical industry and industrial research, catalysis play an important role. Different catalysts are in constant development to fulfil economic, political and environmental demands. When using a catalyst, it is possible to replace a polluting chemical reaction with a more environmentally friendly alternative. Today, and in the future, this can be vital for the chemical industry. In addition, it's important for a company/researcher to pay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Syngas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principly used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline, when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII (in Germany alone half a million cars were built or rebuilt to run on wood gas). Production Syngas is produced by steam reforming or partial oxidation of natural gas or liquid hydrocarbons, or coal gasification. Steam reforming of methane is an endothermic reaction requiring 206 kJ/mol of methane: : In principle, but rarely in practice, biomass and related hydrocarbon feedstocks could be used to generate biogas and biochar in waste-to-energy gasification facilities. The gas generated (mostly methane and carbon dioxide) is sometimes described as ''syngas'' bu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ostwald Process
The Ostwald process is a chemical process used for making nitric acid (HNO3). Wilhelm Ostwald developed the process, and he patented it in 1902. The Ostwald process is a mainstay of the modern chemical industry, and it provides the main raw material for the most common type of fertilizer production. Historically and practically, the Ostwald process is closely associated with the Haber process, which provides the requisite raw material, ammonia (NH3). Description Stage 1 Ammonia is converted to nitric acid in 2 stages. It is oxidized by heating with oxygen in the presence of a catalyst such as platinum with 10% rhodium, platinum metal on fused silica wool, copper or nickel, to form nitric oxide (nitrogen(II) oxide) and water (as steam). This reaction is strongly exothermic, making it a useful heat source once initiated: :4 NH3 (g) + 5 O2 (g) -> 4 NO (g) + 6 H2O (g) (Δ''H'' = −905.2 kJ/mol) Stage 2 Stage two encompasses two reactions and is carried out in an absorption ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "''lime''" connotes calcium-containing inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, ''quicklime'' specifically applies to the single chemical compound calcium oxide. Calcium oxide that survives processing without reacting in building products such as cement is called free lime. Quicklime is relatively inexpensive. Both it and a chemical derivative ( calcium hydroxide, of which quicklime is the base anhydride) are important commodity chemicals. Preparation Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by heating the material to above ,Me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Oxide
Potassium oxide ( K O) is an ionic compound of potassium and oxygen. It is a base. This pale yellow solid is the simplest oxide of potassium. It is a highly reactive compound that is rarely encountered. Some industrial materials, such as fertilizers and cements, are assayed assuming the percent composition that would be equivalent to K2O. Production Potassium oxide is produced from the reaction of oxygen and potassium; this reaction affords potassium peroxide, K2O2. Treatment of the peroxide with potassium produces the oxide: : K2O2 + 2 K -> 2 K2O Alternatively and more conveniently, K2O is synthesized by heating potassium nitrate with metallic potassium: :2KNO3 + 10K -> 6K2O + N2 (^) Other possibility is to heat potassium peroxide at 500 °C which decomposes at that temperature giving pure potassium oxide and oxygen. :2K2O2 -> 2K2O + O2 (^) Potassium hydroxide cannot be further dehydrated to the oxide but it can react with molten potassium to produce it, releasing hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haber–Bosch Process
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and Carl Bosch, who developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using a metal catalyst under high temperatures and pressures: : \ce \quad \Delta H^\circ = -91.8~\text Though this reaction is exothermic (i.e. it releases energy, albeit not very much), it results in a decrease in entropy, which is the central reason why it is very challenging to carry out. Before the development of the Haber process, it had been difficult to produce ammonia on an industrial scale, with early methods, such as the Birkeland–Eyde process and the Frank–Caro process, all highly inefficient. During World War I, the Haber process provided Germany with a sou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. p. 617. In such a process, a closed system usually absorbs thermal energy from its surroundings, which is heat transfer into the system. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. It may be a chemical process, such as dissolving ammonium nitrate () in water (), or a physical process, such as the melting of ice cubes. The term was coined by 19th-century French chemist Marcellin Berthelot. The opposite of an endothermic process is an exothermic process, one that releases or "gives out" energy, usually in the form of heat and sometimes as electrical energy. Thus in each term (endothermic and exothermic) the prefix refers to where heat (or electri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steam Reforming
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen production. The reaction is represented by this equilibrium: :CH4 + H2O CO + 3 H2 The reaction is strongly endothermic (Δ''H''SR = 206 kJ/mol). Hydrogen produced by steam reforming is termed 'grey hydrogen' when the waste carbon monoxide is released to the atmosphere and 'blue hydrogen' when the carbon monoxide is (mostly) captured and stored geologically - see carbon capture and storage. Zero carbon 'green' hydrogen is produced by thermochemical water splitting, using solar thermal, low- or zero-carbon electricity or waste heat, or electrolysis, using low- or zero-carbon electricity. Zero carbon emissions 'turquoise' hydrogen is produced by one-step methane pyrolysis of natural gas. Steam reforming of natural gas produces most of the worl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
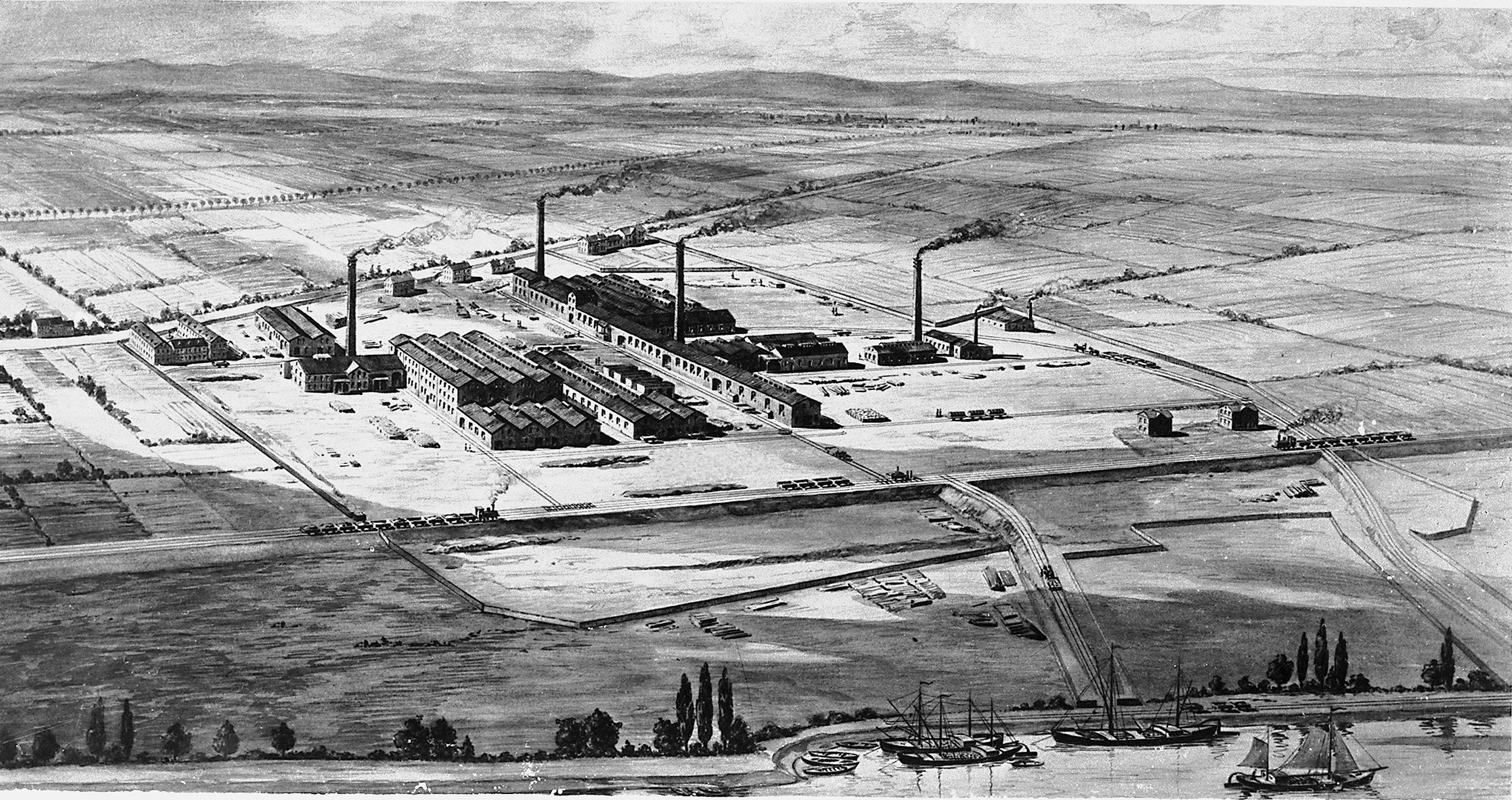
Imperial Chemical Industries
Imperial Chemical Industries (ICI) was a British chemical company. It was, for much of its history, the largest manufacturer in Britain. It was formed by the merger of four leading British chemical companies in 1926. Its headquarters were at Millbank in London. ICI was a constituent of the FT 30 and later the FTSE 100 indices. ICI made general chemicals, plastics, paints, pharmaceuticals and speciality products, including food ingredients, speciality polymers, electronic materials, fragrances and flavourings. In 2008, it was acquired by AkzoNobel, which immediately sold parts of ICI to Henkel and integrated ICI's remaining operations within its existing organisation. History Development of the business (1926–1944) The company was founded in December 1926 from the merger of four companies: Brunner Mond, Nobel Explosives, the United Alkali Company, and British Dyestuffs Corporation. It established its head office at Millbank in London in 1928. Competing with DuPo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BASF
BASF SE () is a German multinational chemical company and the largest chemical producer in the world. Its headquarters is located in Ludwigshafen, Germany. The BASF Group comprises subsidiaries and joint ventures in more than 80 countries and operates six integrated production sites and 390 other production sites in Europe, Asia, Australia, the Americas and Africa. BASF has customers in over 190 countries and supplies products to a wide variety of industries. Despite its size and global presence, BASF has received relatively little public attention since it abandoned the manufacture and sale of BASF-branded consumer electronics products in the 1990s. At the end of 2019, the company employed 117,628 people, with over 54,000 in Germany. , BASF posted sales of €59.3 billion and income from operations before special items of about €4.5 billion. Between 1990 and 2005, the company invested €5.6 billion in Asia, specifically in sites near Nanjing and Shanghai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |