|
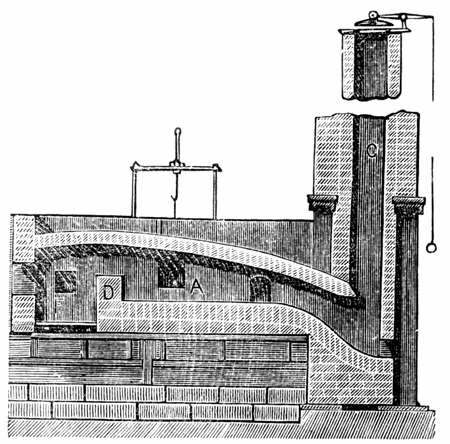
Heaton Process
The Heaton process is one of the 18th century processes for refining pig iron without the use of charcoal. Inventors John Heaton developed this process using nitrates to oxidize the 2-5% carbon in cast iron and convert it to steel. Process In 1869, when John Heaton published 'Heaton's Process for the Treatment of Cast Iron and the Manufacture of Steel', cast iron was a readily available material, but converting it to steel was a slow, expensive, laborious process. At the time, there was an already-known laboratory-scale process of adding "nitre" to cast iron in order to produce oxygen and burn off the carbon, producing steel. John Heaton formalized a process (specifying sodium nitrate instead of potassium) and designed equipment which made the comingling of the nitrate and the liquid cast iron reliable and repeatable, something that had until then been impractical. Combined, these improvements became the Heaton process. Another English metallurgist, Henry Bessemer had just created ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
John Heaton (metallurgist)
John Heaton (1818–1897) was a 19th-century mechanical engineer who lived in England. Government documents refer to him at different times as a civil, mechanical and mining engineer, reflecting the breadth of his work. He is mainly known for inventing the Heaton process, a way to convert cast iron into steel, and his subsequent legal battles with fellow Englishman Henry Bessemer, who had developed a different method for the same conversion. He later obtained a patent regarding efficiency improvements in steam boilers. Life * The Nottinghamshire Birth-Marriage-Death records show he was born (or baptised) in 1818. * In 1868 he received a UK patent for the Heaton process. * In 1868 he was nominated for membership of the Institute of Mechanical Engineers and was then accepted. * Between 1868 and 1869 he conducted, and won, a protracted legal battle with Henry Bessemer. Bessemer believed that the Heaton process was included in the Bessemer process through some early patent applicatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrate
Nitrate is a polyatomic ion with the chemical formula . Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble nitrate is bismuth oxynitrate. Structure The ion is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a − charge, whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion. This arrangement is commonly used as an example of resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by resonance structures: Dietary nitrate A rich source of inorganic nitrate in the human diets come from leafy green foo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cast Iron
Cast iron is a class of iron– carbon alloys with a carbon content more than 2%. Its usefulness derives from its relatively low melting temperature. The alloy constituents affect its color when fractured: white cast iron has carbide impurities which allow cracks to pass straight through, grey cast iron has graphite flakes which deflect a passing crack and initiate countless new cracks as the material breaks, and ductile cast iron has spherical graphite "nodules" which stop the crack from further progressing. Carbon (C), ranging from 1.8 to 4 wt%, and silicon (Si), 1–3 wt%, are the main alloying elements of cast iron. Iron alloys with lower carbon content are known as steel. Cast iron tends to be brittle, except for malleable cast irons. With its relatively low melting point, good fluidity, castability, excellent machinability, resistance to deformation and wear resistance, cast irons have become an engineering material with a wide range of applications and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant typically need an additional 11% chromium. Because of its high tensile strength and low cost, steel is used in buildings, infrastructure, tools, ships, trains, cars, machines, electrical appliances, weapons, and rockets. Iron is the base metal of steel. Depending on the temperature, it can take two crystalline forms (allotropic forms): body-centred cubic and face-centred cubic. The interaction of the allotropes of iron with the alloying elements, primarily carbon, gives steel and cast iron their range of unique properties. In pure iron, the crystal structure has relatively little resistance to the iron atoms slipping past one another, and so pure iron is quite ductile, or soft and easily formed. In steel, small amounts of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Nitrate
Potassium nitrate is a chemical compound with the chemical formula . This alkali metal nitrate salt is also known as Indian saltpetre (large deposits of which were historically mined in India). It is an ionic salt of potassium ions K+ and nitrate ions NO3−, and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter (or ''nitre'' in the UK). It is a source of nitrogen, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpeter (or ''saltpetre'' in the UK). Major uses of potassium nitrate are in fertilizers, tree stump removal, rocket propellants and fireworks. It is one of the major constituents of gunpowder (black powder). In processed meats, potassium nitrate reacts with hemoglobin and myoglobin generating a red color. Etymology Potassium nitrate, because of its early and global use and production, has many names. Hebrew and Egyptian words for it had the co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Nitrate
Sodium nitrate is the chemical compound with the formula . This alkali metal nitrate salt is also known as Chile saltpeter (large deposits of which were historically mined in Chile) to distinguish it from ordinary saltpeter, potassium nitrate. The mineral form is also known as nitratine, nitratite or soda niter. Sodium nitrate is a white deliquescent solid very soluble in water. It is a readily available source of the nitrate anion (NO3−), which is useful in several reactions carried out on industrial scales for the production of fertilizers, pyrotechnics, smoke bombs and other explosives, glass and pottery enamels, food preservatives (esp. meats), and solid rocket propellant. It has been mined extensively for these purposes. History The first shipment of saltpeter to Europe arrived in England from Peru in 1820 or 1825, right after that country's independence from Spain, but did not find any buyers and was dumped at sea in order to avoid customs toll.Friedrich Georg Wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys. Metallurgy encompasses both the science and the technology of metals; that is, the way in which science is applied to the production of metals, and the engineering of metal components used in products for both consumers and manufacturers. Metallurgy is distinct from the craft of metalworking. Metalworking relies on metallurgy in a similar manner to how medicine relies on medical science for technical advancement. A specialist practitioner of metallurgy is known as a metallurgist. The science of metallurgy is further subdivided into two broad categories: chemical metallurgy and physical metallurgy. Chemical metallurgy is chiefly concerned with the reduction and oxidation of metals, and the chemical performance of metals. Subjects of study in chemical metallurgy include m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Henry Bessemer
Sir Henry Bessemer (19 January 1813 – 15 March 1898) was an English inventor, whose steel-making process would become the most important technique for making steel in the nineteenth century for almost one hundred years from 1856 to 1950. He also played a significant role in establishing the town of Sheffield, nicknamed ‘Steel City’, as a major industrial centre. Bessemer had been trying to reduce the cost of steel-making for military ordnance, and developed his system for blowing air through molten pig iron to remove the impurities. This made steel easier, quicker and cheaper to manufacture, and revolutionised structural engineering. One of the most significant inventors of the Second Industrial Revolution, Bessemer also made over 100 other inventions in the fields of iron, steel and glass. Unlike many inventors, he managed to bring his own projects to fruition and profited financially from their success. He was knighted for his contribution to science in 1879, and in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bessemer Process
The Bessemer process was the first inexpensive industrial process for the mass production of steel from molten pig iron before the development of the open hearth furnace. The key principle is removal of impurities from the iron by oxidation with air being blown through the molten iron. The oxidation also raises the temperature of the iron mass and keeps it molten. Related decarburizing with air processes had been used outside Europe for hundreds of years, but not on an industrial scale. One such process (similar to puddling) was known in the 11th century in East Asia, where the scholar Shen Kuo of that era described its use in the Chinese iron and steel industry. In the 17th century, accounts by European travelers detailed its possible use by the Japanese. The modern process is named after its inventor, the Englishman Henry Bessemer, who took out a patent on the process in 1856. The process was said to be independently discovered in 1851 by the American inventor Willi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Puddling (metallurgy)
Puddling is the process of converting pig iron to bar (wrought) iron in a coal fired reverberatory furnace. It was developed in England during the 1780s. The molten pig iron was stirred in a reverberatory furnace, in an oxidizing environment, resulting in wrought iron. It was one of the most important processes of making the first appreciable volumes of valuable and useful bar iron (malleable wrought iron) without the use of charcoal. Eventually, the furnace would be used to make small quantities of specialty steels. Though it was not the first process to produce bar iron without charcoal, puddling was by far the most successful, and replaced the earlier potting and stamping processes, as well as the much older charcoal finery and bloomery processes. This enabled a great expansion of iron production to take place in Great Britain, and shortly afterwards, in North America. That expansion constitutes the beginnings of the Industrial Revolution so far as the iron industry is co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potting And Stamping
Potting and stamping is a modern name for one of the 18th-century processes for refining pig iron without the use of charcoal. Inventors The process was devised by Charles Wood of Lowmill, Egremont in Cumberland and his brother John Wood of Wednesbury and patented by them in 1761 and 1763. The process was improved by John Wright and Joseph Jesson of West Bromwich, who also obtained a patent. Process The process involved the melting of pig iron in an oxidising atmosphere. The metal was then allowed to cool, broken up by stamping, and washed. The granulated iron was then heated in pots in a reverberatory furnace. The resultant bloom was then drawn out under a forge hammer in the usual way. Adoption During the 14-year term of the patents, the process was little used except by the inventors. However, from c.1785, shortly before Wright & Jesson's process came out of patent, it seems to have been adopted by many ironmasters in the West Midlands. Professor Charles Hyde argues th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |