|
Fréedericksz Transition
The Fréedericksz transition is a phase transition in liquid crystals produced when a sufficiently strong electric or magnetic field is applied to a liquid crystal in an undistorted state. Below a certain field threshold the director remains undistorted. As the field value is gradually increased from this threshold, the director begins to twist until it is aligned with the field. In this fashion the Fréedericksz transition can occur in three different configurations known as the twist, bend, and splay geometries. The phase transition was first observed by Fréedericksz and Repiewa in 1927. In this first experiment of theirs, one of the walls of the cell was concave so as to produce a variation in thickness along the cell. The phase transition is named in honor of the Russian physicist Vsevolod Frederiks. Derivation Twist geometry If a nematic liquid crystal that is confined between two parallel plates that induce a planar anchoring is placed in a sufficiently high constant electri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic State of matter, states of matter: solid, liquid, and gas, and in rare cases, plasma (physics), plasma. A phase of a thermodynamic system and the states of matter have uniform physical property, physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition States of matter Phase transitions commonly refer to when a substance tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
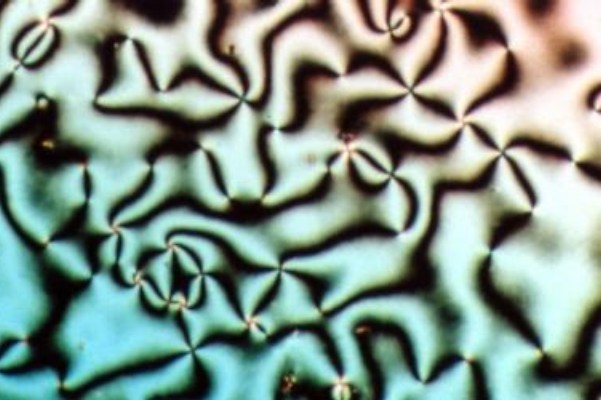
Liquid Crystals
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in a solid. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter (just as water may be ice or water vapour). Liquid crystals can be divided into three main types: thermotropic, lyotropic, and metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Field
An electric field (sometimes called E-field) is a field (physics), physical field that surrounds electrically charged particles such as electrons. In classical electromagnetism, the electric field of a single charge (or group of charges) describes their capacity to exert attractive or repulsive forces on another charged object. Charged particles exert attractive forces on each other when the sign of their charges are opposite, one being positive while the other is negative, and repel each other when the signs of the charges are the same. Because these forces are exerted mutually, two charges must be present for the forces to take place. These forces are described by Coulomb's law, which says that the greater the magnitude of the charges, the greater the force, and the greater the distance between them, the weaker the force. Informally, the greater the charge of an object, the stronger its electric field. Similarly, an electric field is stronger nearer charged objects and weaker f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Field
A magnetic field (sometimes called B-field) is a physical field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to the magnetic field. A permanent magnet's magnetic field pulls on ferromagnetic materials such as iron, and attracts or repels other magnets. In addition, a nonuniform magnetic field exerts minuscule forces on "nonmagnetic" materials by three other magnetic effects: paramagnetism, diamagnetism, and antiferromagnetism, although these forces are usually so small they can only be detected by laboratory equipment. Magnetic fields surround magnetized materials, electric currents, and electric fields varying in time. Since both strength and direction of a magnetic field may vary with location, it is described mathematically by a function (mathematics), function assigning a Euclidean vector, vector to each point of space, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Crystal
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in a solid. There are many types of LC Phase (matter), phases, which can be distinguished by their Optics, optical properties (such as Texture (crystalline), textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter (just as water may be ice or water vapour). Liquid crystals can be divided into three main types: thermotropic, lyotropic, and #Metallotropic liquid crystals, metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vsevolod Frederiks
Vsevolod Konstantinovich Frederiks (or Fréedericksz; ; April 29, 1885, Warsaw – January 6, 1944, Gorkiy) was a Russian/Soviet physicist. His primary contribution was in the field of liquid crystals. The Frederiks transition is named after him. After high school, Frederiks attended Geneva University and attended the lectures of Paul Langevin in Paris for one semester. After defending his thesis and obtaining his PhD, Frederiks decided to continue his studies at Göttingen University. He was there for more than eight years, and with the outbreak of World War I he became a civil prisoner. During that period, he became personal assistant to David Hilbert. In the summer of 1918, Frederiks returned to Russia, and worked at the Institute of Physics and Biophysics in Moscow. In 1919, he became a lecturer at the University of Petrograd. He was arrested by the NKVD in 1937. Although released before World War II, he died before reaching home.Elizabeth Wilson. ''Shostakovich: A Life Remem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distortion Free Energy Density
The distortion free energy density is a quantity that describes the increase in the free energy density of a liquid crystal caused by distortions from its uniformly aligned configuration. It also commonly goes by the name Frank free energy density named after Charles Frank. Nematic liquid crystal The distortion free energy density in a nematic liquid crystal is a measure of the increase in the Helmholtz free energy In thermodynamics, the Helmholtz free energy (or Helmholtz energy) is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature ( isothermal). The change in the Helmholtz ene ... per unit volume due to deviations in the orientational ordering away from a uniformly aligned nematic director configuration. The total free energy density for a nematic is therefore given by: :\mathcal_=\mathcal_+\mathcal_ , where \mathcal_ is the total free energy density of a liquid crystal, \mathcal_ is the free ener ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calculus Of Variations
The calculus of variations (or variational calculus) is a field of mathematical analysis that uses variations, which are small changes in Function (mathematics), functions and functional (mathematics), functionals, to find maxima and minima of functionals: Map (mathematics), mappings from a set of Function (mathematics), functions to the real numbers. Functionals are often expressed as definite integrals involving functions and their derivatives. Functions that maximize or minimize functionals may be found using the Euler–Lagrange equation of the calculus of variations. A simple example of such a problem is to find the curve of shortest length connecting two points. If there are no constraints, the solution is a straight line between the points. However, if the curve is constrained to lie on a surface in space, then the solution is less obvious, and possibly many solutions may exist. Such solutions are known as ''geodesics''. A related problem is posed by Fermat's principle: li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complete Elliptic Integral Of The First Kind
In integral calculus, an elliptic integral is one of a number of related functions defined as the value of certain integrals, which were first studied by Giulio Carlo de' Toschi di Fagnano, Giulio Fagnano and Leonhard Euler (). Their name originates from their originally arising in connection with the problem of finding the arc length of an ellipse. Modern mathematics defines an "elliptic integral" as any function (mathematics), function which can be expressed in the form f(x) = \int_^ R \, dt, where is a rational function of its two arguments, is a polynomial of degree 3 or 4 with no repeated roots, and is a constant. In general, integrals in this form cannot be expressed in terms of elementary functions. Exceptions to this general rule are when has repeated roots, or when contains no odd powers of or if the integral is pseudo-elliptic. However, with the appropriate Integration by reduction formulae, reduction formula, every elliptic integral can be brought into a form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frank Constant
The distortion free energy density is a quantity that describes the increase in the free energy density of a liquid crystal caused by distortions from its uniformly aligned configuration. It also commonly goes by the name Frank free energy density named after Charles Frank. Nematic liquid crystal The distortion free energy density in a nematic liquid crystal is a measure of the increase in the Helmholtz free energy In thermodynamics, the Helmholtz free energy (or Helmholtz energy) is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature ( isothermal). The change in the Helmholtz ene ... per unit volume due to deviations in the orientational ordering away from a uniformly aligned nematic director configuration. The total free energy density for a nematic is therefore given by: :\mathcal_=\mathcal_+\mathcal_ , where \mathcal_ is the total free energy density of a liquid crystal, \mathcal_ is the free ener ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Crystals
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in a solid. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter (just as water may be ice or water vapour). Liquid crystals can be divided into three main types: thermotropic, lyotropic, and metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |