|
Double-layer Capacitance
Double-layer capacitance is the important characteristic of the electrical double layer which appears, for example, at the interface between a conductive electrode and an adjacent liquid electrolyte. At this boundary two layers of charge with opposing polarity form, one at the surface of the electrode, and one in the electrolyte. These two layers, electrons on the electrode and ions in the electrolyte, are typically separated by a single layer of solvent molecules that adhere to the surface of the electrode and act like a dielectric in a conventional capacitor. The amount of electric charge stored in double-layer capacitor depends on the applied voltage. The unit of capacitance is the farad. The double-layer capacitance is the physical principle behind the electrostatic double-layer type of Supercapacitors. History * Development of the double layer and pseudocapacitance model see Double layer (interfacial) * Development of the electrochemical components see Supercapacitors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
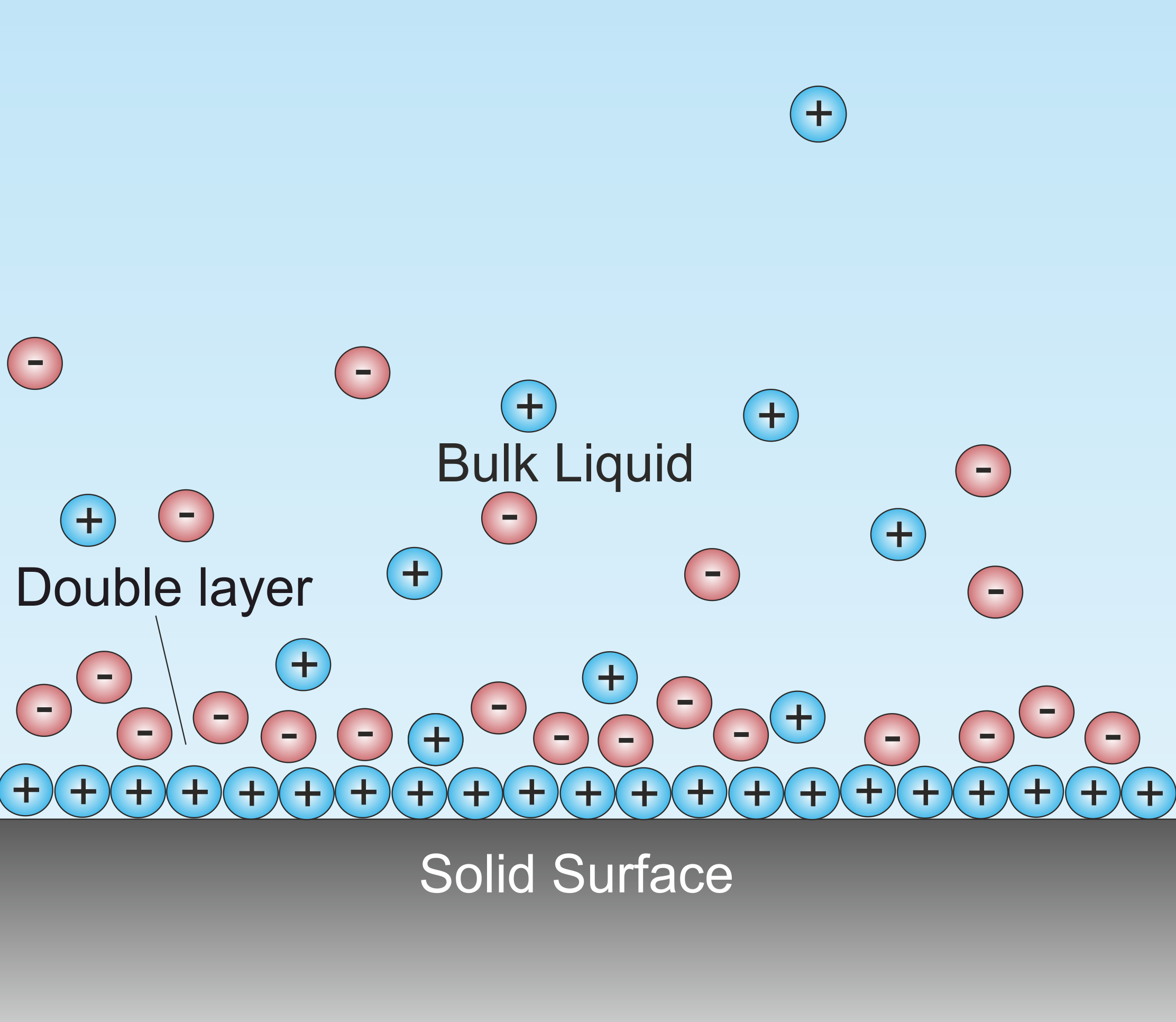
Double Layer (interfacial)
A double layer (DL, also called an electrical double layer, EDL) is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge (either positive or negative), consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer". Interfacial DLs are most apparent in systems with a large surface area to volume ratio, such as a colloid or porous bodies with particles or pores (respectively) on th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a fluid (the ''absorbate'') is dissolved by or permeates a liquid or solid (the ''absorbent''). Adsorption is a '' surface phenomenon'', while absorption involves the whole volume of the material, although adsorption does often precede absorption. The term '' sorption'' encompasses both processes, while '' desorption'' is the reverse of it. Like surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent or metallic) of the constituent atoms of the material are fulfilled by other atoms in the material. However, atoms on the surface of the adsorbent are not wholly surrounded by other adsorbent atoms and therefore can attract adsorbates. The exact nature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
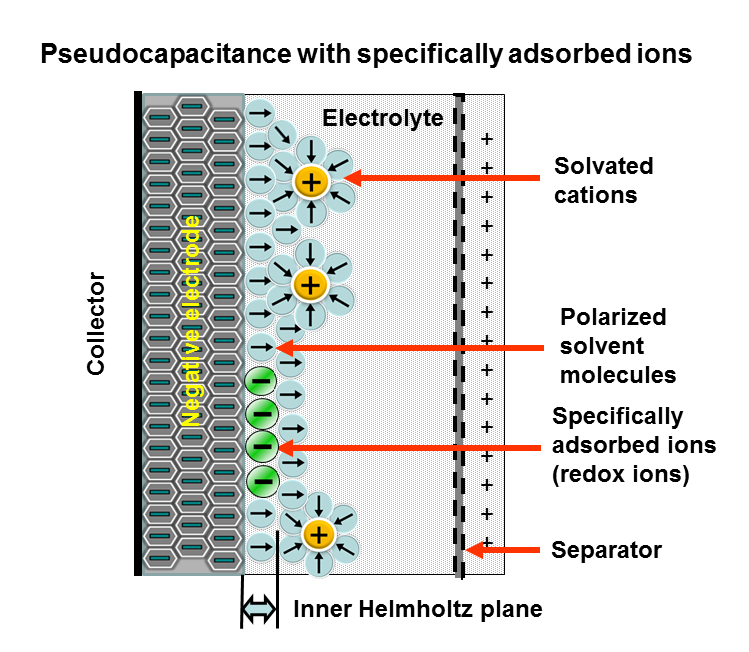
Pseudocapacitance
Pseudocapacitance is the Electrochemistry, electrochemical storage of electricity in an Supercapacitor, electrochemical capacitor (Pseudocapacitor). This faradaic charge transfer originates by a very fast sequence of reversible Faradaic current, faradaic redox, Capacitive deionization, electrosorption or Intercalation (chemistry), intercalation processes on the surface of suitable electrodes. see alsBrian E. Conway in Electrochemistry Encyclopedia: ''ELECTROCHEMICAL CAPACITORS Their Nature, Function, and Applications''E. Frackowiak, F. Beguin: ''Carbon Materials For The Electrochemical Storage Of Energy In Capacitors.'' In: ''CARBON.'' 39, 2001, S. 937–950PDF E. Frackowiak, K. Jurewicz, S. Delpeux, F. Béguin: ''Nanotubular Materials For Supercapacitors.'' In: ''Journal of Power Sources.'' Volumes 97–98, Juli 2001, S. 822–825, . Pseudocapacitance is accompanied by an electron Charge transfer complex, charge-transfer between electrolyte and electrode coming from a Solvation, de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ångström
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.merriam-webster.com/dictionary/angstrom. (, ; , ) or ångström is a metric unit of length equal to m; that is, one ten-billionth ( US) of a metre, a hundred-millionth of a centimetre,Entry "angstrom" in the Oxford English Dictionary, 2nd edition (1986). Retrieved on 2021-11-22 from https://www.oed.com/oed2/00008552. 0.1 nanometre, or 100 picometres. Its symbol is Å, a letter of the Swedish alphabet. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–1874). The angstrom is often used in the natural sciences and technology to express sizes of atoms, molecules, microscopic biological structures, and lengths of chemical bonds, arrangement of atoms in crystals,Arturas Vailionis (2015):Geometry of Crystals Le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Permittivity
In electromagnetism, the absolute permittivity, often simply called permittivity and denoted by the Greek letter ''ε'' (epsilon), is a measure of the electric polarizability of a dielectric. A material with high permittivity polarizes more in response to an applied electric field than a material with low permittivity, thereby storing more energy in the material. In electrostatics, the permittivity plays an important role in determining the capacitance of a capacitor. In the simplest case, the electric displacement field D resulting from an applied electric field E is :\mathbf = \varepsilon \mathbf. More generally, the permittivity is a thermodynamic function of state. It can depend on the frequency, magnitude, and direction of the applied field. The SI unit for permittivity is farad per meter (F/m). The permittivity is often represented by the relative permittivity ''ε''r which is the ratio of the absolute permittivity ''ε'' and the vacuum permittivity ''ε''0 :\kapp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Differential Capacitance
Differential capacitance in physics, electronics, and electrochemistry is a measure of the voltage-dependent capacitance of a nonlinear capacitor, such as an electrical double layer or a semiconductor diode. It is defined as the derivative of charge with respect to potential. Description In electrochemistry differential capacitance is a parameter introduced for characterizing electrical double layers: : C = \frac where σ is surface charge and ψ is electric surface potential. Capacitance is usually defined as the stored charge between two conducting surfaces separated by a dielectric divided by the voltage between the surfaces. Another definition is the rate of change of the stored charge or surface charge (σ) divided by the rate of change of the voltage between the surfaces or the electric surface potential (ψ). The latter is called the "differential capacitance," but usually the stored charge is directly proportional to the voltage, making the capacitances given by the two ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Breakdown
Electrical breakdown or dielectric breakdown is a process that occurs when an electrical insulating material, subjected to a high enough voltage, suddenly becomes an electrical conductor and electric current flows through it. All insulating materials undergo breakdown when the electric field caused by an applied voltage exceeds the material's dielectric strength. The voltage at which a given insulating object becomes conductive is called its breakdown voltage and in addition to its dielectric strength depends on its size and shape, and the location on the object at which the voltage is applied. Under sufficient electrical potential, electrical breakdown can occur within solids, liquids, or gases (and theoretically even in a vacuum). However, the specific breakdown mechanisms are different for each kind of dielectric medium. Electrical breakdown may be a momentary event (as in an electrostatic discharge), or may lead to a continuous electric arc if protective devices fail to int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Field Strength
In physics, field strength means the ''magnitude'' of a vector-valued field (e.g., in volts per meter, V/m, for an electric field ''E''). For example, an electromagnetic field results in both electric field strength and magnetic field strength. As an application, in radio frequency telecommunications Telecommunication is the transmission of information by various types of technologies over wire, radio, optical, or other electromagnetic systems. It has its origin in the desire of humans for communication over a distance greater than that fe ..., the signal strength excites a receiving antenna and thereby induces a voltage at a specific frequency and polarization in order to provide an input signal to a radio receiver. Field strength meters are used for such applications as cellular, broadcasting, wi-fi and a wide variety of other radio-related applications. See also * Dipole field strength in free space * Field strength tensor * Signal strength in telecommunications ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolytic Capacitors
An electrolytic capacitor is a polarized capacitor whose anode or positive plate is made of a metal that forms an insulating oxide layer through anodization. This oxide layer acts as the dielectric of the capacitor. A solid, liquid, or gel electrolyte covers the surface of this oxide layer, serving as the cathode or negative plate of the capacitor. Due to their very thin dielectric oxide layer and enlarged anode surface, electrolytic capacitors have a much higher capacitance-voltage (CV) product per unit volume than ceramic capacitors or film capacitors, and so can have large capacitance values. There are three families of electrolytic capacitor: aluminum electrolytic capacitors, tantalum electrolytic capacitors, and niobium electrolytic capacitors. The large capacitance of electrolytic capacitors makes them particularly suitable for passing or bypassing low-frequency signals, and for storing large amounts of energy. They are widely used for decoupling or noise filter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Debye Length
In plasmas and electrolytes, the Debye length \lambda_ (also called Debye radius), is a measure of a charge carrier's net electrostatic effect in a solution and how far its electrostatic effect persists. With each Debye length the charges are increasingly electrically screened and the electric potential decreases in magnitude by 1/ e. A Debye sphere is a volume whose radius is the Debye length. Debye length is an important parameter in plasma physics, electrolytes, and colloids ( DLVO theory). The corresponding Debye screening wave vector k_=1/\lambda_ for particles of density n, charge q at a temperature T is given by k_^2=4\pi n q^2/(k_T) in Gaussian units. Expressions in MKS units will be given below. The analogous quantities at very low temperatures (T \to 0) are known as the Thomas–Fermi length and the Thomas–Fermi wave vector. They are of interest in describing the behaviour of electrons in metals at room temperature. The Debye length is named after the Dutch-Ame ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |