|
Dystrophin
Dystrophin is a rod-shaped cytoplasmic protein, and a vital part of a protein complex that connects the cytoskeleton of a muscle fiber to the surrounding extracellular matrix through the cell membrane. This complex is variously known as the costamere or the dystrophin-associated protein complex (DAPC). Many muscle proteins, such as α-dystrobrevin, syncoilin, synemin, sarcoglycan, dystroglycan, and sarcospan, colocalize with dystrophin at the costamere. It has a molecular weight of 427 kDa. Dystrophin is coded for by the ''DMD'' gene – the largest known human gene, covering 2.4 megabases (0.08% of the human genome) at Locus (genetics), locus X chromosome, Xp21. The primary transcript in muscle measures about 2,100 kilobases and takes 16 hours to transcribe; the Mature messenger RNA, mature mRNA measures 14.0 kilobases. The 79-exon muscle transcript codes for a protein of 3685 amino acid residues. Spontaneous or inherited mutations in the dystrophin gene can cause different form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dystrobrevin
Dystrobrevin is a protein that binds to dystrophin in the costamere of skeletal muscle cells. In humans, there are at least two isoforms of dystrobrevin, dystrobrevin alpha and dystrobrevin beta. Dystrobrevins are members of dystrophin-related protein family which are thought to play an important role in intracellular signal transduction and provide a membrane scaffold in muscle. Defects in dystrobrevins and their associated proteins cause a range of neuromuscular diseases such as muscular dystrophies. Dystrobrevin was first identified by isolating from the electric organ of the electric ray '' Torpedo californica.'' It is a phosphoprotein, which weights 87 kDa, associated with the postsynaptic membrane at the cytoplasmic face. Dystrobrevin proteins have been said to participates in the formation and stability of synapses because it copurifies with acetylcholine receptors from ''Torpedo'' electric organ membranes. In 1997, an experiment was done using the yeast two-hybrid m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Duchenne Muscular Dystrophy
Duchenne muscular dystrophy (DMD) is a severe type of muscular dystrophy predominantly affecting boys. The onset of muscle weakness typically begins around age four, with rapid progression. Initially, muscle loss occurs in the thighs and pelvis, extending to the arms, which can lead to difficulties in standing up. By the age of 12, most individuals with Duchenne muscular dystrophy are unable to walk. Affected muscles may appear larger due to an increase in fat content, and scoliosis is common. Some individuals may experience intellectual disability, and females carrying a single copy of the mutated gene may show mild symptoms. Duchenne muscular dystrophy is caused by mutations or deletions in any of the 79 exons encoding the large dystrophin protein, which is essential for maintaining the muscle fibers' cell membrane integrity. The disorder follows an X-linked recessive inheritance pattern, with approximately two-thirds of cases inherited from the mother and one-third res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dystrophin-associated Protein Complex
The dystrophin-associated protein complex, also known as the dystrophin-associated glycoprotein complex is a multiprotein complex that includes dystrophin and the dystrophin-associated proteins. It is one of the two protein complexes that make up the costamere in striated muscle cells. The other complex is the ''integrin-vinculin-talin complex''. Structure The dystrophin-associated protein complex includes dystrophin. Dystrophin binds to actin of the cytoskeleton, and also to proteins in the extracellular matrix. The dystrophin-associated protein complex also contains dystrophin-associated proteins. This includes a four subunit sarcoglycan complex, which is fixed to dystrophin in muscle cells. In the epithelia of the kidney, dystrophin may be replaced with utrophin. Aquaporin 4 may be connected to the dystrophin-associated protein complex. Function The dystrophin-associated protein complex is important for cell structure and cell signalling. It is one of two protein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dystroglycan
Dystroglycan is a protein that in humans is encoded by the ''DAG1'' gene. Dystroglycan is one of the dystrophin-associated glycoproteins, which is encoded by a 5.5 kb transcript in ''Homo sapiens'' on chromosome 3. There are two exons that are separated by a large intron. The spliced exons code for a protein product that is finally cleaved into two non-covalently associated subunits, lpha(N-terminal) and eta(C-terminal). Function In skeletal muscle the dystroglycan complex works as a transmembrane linkage between the extracellular matrix and the cytoskeleton. lphadystroglycan is extracellular and binds to merosin lpha2 laminin in the basement membrane, while etadystroglycan is a transmembrane protein and binds to dystrophin, which is a large rod-like cytoskeletal protein, absent in Duchenne muscular dystrophy patients. Dystrophin binds to intracellular actin cables. In this way, the dystroglycan complex, which links the extracellular matrix to the intracellular actin c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarcospan
Sarcospan is a protein that in humans is encoded by the SSPN gene. Originally identified as Kirsten ras associated gene (KRAG), sarcospan is a 25-kDa transmembrane protein located in the dystrophin-associated protein complex of skeletal muscle cells, where it is most abundant. It contains four transmembrane spanning helices with both N- and C-terminal domains located intracellularly. Loss of SSPN expression occurs in patients with Duchenne muscular dystrophy. Dystrophin is required for proper localization of SSPN. SSPN is also an essential regulator of Akt/PKB signaling pathway, Akt signaling pathways. Without SSPN, Akt signaling pathways will be hindered and muscle regeneration will not occur. Function Sarcospan is a protein that plays a crucial role in muscle health and function. It is part of the dystrophin-associated glycoprotein complex (DGC), which is a protein complex found in muscle cells that helps to maintain the structural integrity of muscle fibers. Sarcospan interac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dystroglycan
Dystroglycan is a protein that in humans is encoded by the ''DAG1'' gene. Dystroglycan is one of the dystrophin-associated glycoproteins, which is encoded by a 5.5 kb transcript in ''Homo sapiens'' on chromosome 3. There are two exons that are separated by a large intron. The spliced exons code for a protein product that is finally cleaved into two non-covalently associated subunits, lpha(N-terminal) and eta(C-terminal). Function In skeletal muscle the dystroglycan complex works as a transmembrane linkage between the extracellular matrix and the cytoskeleton. lphadystroglycan is extracellular and binds to merosin lpha2 laminin in the basement membrane, while etadystroglycan is a transmembrane protein and binds to dystrophin, which is a large rod-like cytoskeletal protein, absent in Duchenne muscular dystrophy patients. Dystrophin binds to intracellular actin cables. In this way, the dystroglycan complex, which links the extracellular matrix to the intracellular actin c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Costamere
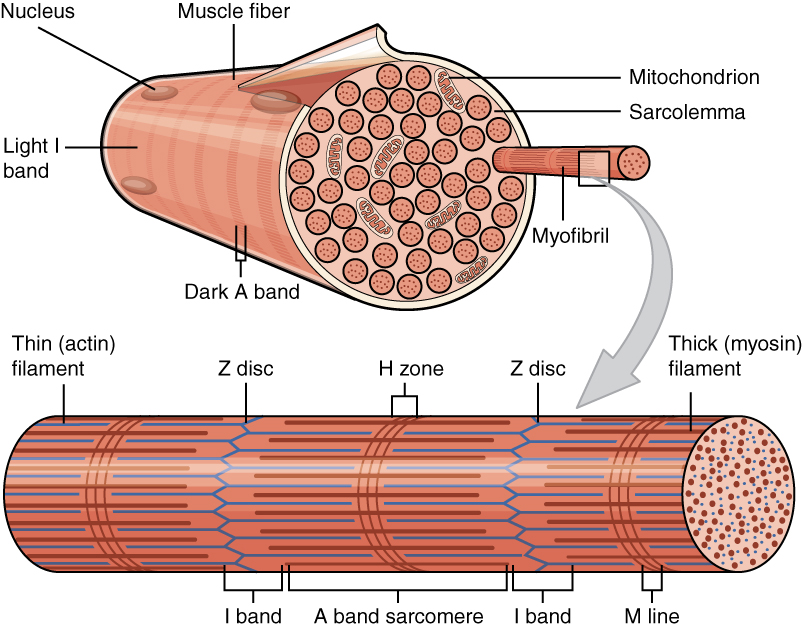
The costamere is a structural-functional component of striated muscle cells which connects the sarcomere of the muscle to the cell membrane (i.e. the sarcolemma).20: 2327-2331 Costameres are sub-sarcolemmal protein assemblies circumferentially aligned in register with the Z-disk of peripheral myofibrils. They physically couple force-generating sarcomeres with the sarcolemma in striated muscle cells and are thus considered one of several "Achilles' heels" of skeletal muscle, a critical component of striated muscle morphology which, when compromised, is thought to directly contribute to the development of several distinct myopathies. The dystrophin-associated protein complex, also referred to as the dystrophin-associated glycoprotein complex (DGC or DAGC), contains various integral and peripheral membrane proteins such as dystroglycans and sarcoglycans, which are thought to be responsible for linking the internal cytoskeletal system of individual myofibers to structural protei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Muscular Dystrophy
Muscular dystrophies (MD) are a genetically and clinically heterogeneous group of rare neuromuscular diseases that cause progressive weakness and breakdown of skeletal muscles over time. The disorders differ as to which muscles are primarily affected, the degree of weakness, how fast they worsen, and when symptoms begin. Some types are also associated with problems in other human organs, organs. Over 30 different disorders are classified as muscular dystrophies. Of those, Duchenne muscular dystrophy (DMD) accounts for approximately 50% of cases and affects males beginning around the age of four. Other relatively common muscular dystrophies include Becker muscular dystrophy, facioscapulohumeral muscular dystrophy, and myotonic dystrophy, whereas limb–girdle muscular dystrophy and congenital muscular dystrophy are themselves groups of several – usually extremely rare – genetic disorders. Muscular dystrophies are caused by mutations in genes, usually those involved in making ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Syncoilin
Discovery Syncoilin is a muscle-specific atypical Intermediate filament#Type III, type III intermediate filament protein encoded in the human by the gene ''SYNC''. It was first isolated as a binding partner to DTNA, α-dystrobrevin, as determined by a yeast two-hybrid assay. Later, a yeast two-hybrid method was used to demonstrate that syncoilin is a binding partner of desmin. These binding partners suggest that syncoilin acts as a mechanical "linker" between the sarcomere Z-disk (where desmin is localized) and the dystrophin-associated protein complex (where α-dystrobrevin is localized). However, the specific ''in vivo'' functions of syncoilin have not yet been determined. Through the use of Western blotting techniques, a second species of syncoilin was found. This species was 55kDa in size, whereas the original species of syncoilin was 64kDa in size. This discovery inspired scientists to use gene splicing to identify two new isoforms called SYNC2 and SYNC3. Abnormally high ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myofilament
Myofilaments are the three protein filaments of myofibrils in muscle cells. The main proteins involved are myosin, actin, and titin. Myosin and actin are the ''contractile proteins'' and titin is an elastic protein. The myofilaments act together in muscle contraction, and in order of size are a thick one of mostly myosin, a thin one of mostly actin, and a very thin one of mostly titin. Types of muscle tissue are striated skeletal muscle and cardiac muscle, obliquely striated muscle (found in some invertebrates), and non-striated smooth muscle. Various arrangements of myofilaments create different muscles. Striated muscle has transverse bands of filaments. In obliquely striated muscle, the filaments are staggered. Smooth muscle has irregular arrangements of filaments. Structure There are three different types of myofilaments: thick, thin, and elastic filaments. *Thick filaments consist primarily of a type of myosin, a motor protein – myosin II. Each thick filament is appr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarcoglycan
The sarcoglycans are a family of transmembrane proteins (α, β, γ, δ or ε) involved in the protein complex responsible for connecting the muscle fibre cytoskeleton to the extracellular matrix, preventing damage to the muscle fibre sarcolemma through shearing forces. The dystrophin Dystrophin is a rod-shaped cytoplasmic protein, and a vital part of a protein complex that connects the cytoskeleton of a muscle fiber to the surrounding extracellular matrix through the cell membrane. This complex is variously known as the costa ... glycoprotein complex (DGC) is a membrane-spanning complex that links the interior cytoskeleton to the extracellular matrix in muscle. The sarcoglycan complex is a subcomplex within the DGC and is composed of six muscle-specific, transmembrane proteins (alpha-, beta-, gamma-, delta-, epsilon-,and zeta-sarcoglycan). The sarcoglycans are asparagine-linked glycosylated proteins with single transmembrane domains. The disorders caused by the mutations of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synemin
Synemin, also known as desmuslin, is a protein that in humans is encoded by the ''SYNM'' gene. Synemin is an intermediate filament (IF) family member. IF proteins are cytoskeletal proteins that confer resistance to mechanical stress and are encoded by a dispersed multigene family. This protein has been found to form a linkage between desmin, which is a subunit of the IF network, and the extracellular matrix, and provides an important structural support in muscle. Function Synemin is an intermediate filament (IF) and, like other IFs, primarily functions to integrate mechanical stress and maintain structural integrity in eukaryotic cells. While it has been observed in a variety of cell types, it has been best studied in the sarcomere of skeletal myocytes. It localizes at the Z-disk and has been shown to bind to α-dystrobrevin, α-actinin, and desmin to act as a mechanical linker in transmitting force laterally throughout the tissue, especially between the contractile myofibrils ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |