|
Chemical Garden
Comparison of chemical gardens grown by NASA scientists on the International Space Station (left) and on the ground (right) A chemical garden while growing up Cobalt(II) chloride upA chemical garden A chemical garden is a set of complex biological-looking structures created by mixing inorganic chemicals. Chemical gardening is an experiment in chemistry usually performed by adding metal salts, such as copper sulfate or cobalt(II) chloride, to an aqueous solution of sodium silicate (otherwise known as waterglass). This results in the growth of plant-like forms in minutes to hours. The chemical garden was first observed and described by Johann Rudolf Glauber in 1646. In its original form, the chemical garden involved the introduction of ferrous chloride (FeCl2) crystals into a solution of potassium silicate (K2SiO3). Process The chemical garden relies on most transition metal silicates being insoluble in water and colored. When a metal salt, such as cobalt chloride, is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Garden
Comparison of chemical gardens grown by NASA scientists on the International Space Station (left) and on the ground (right) A chemical garden while growing up Cobalt(II) chloride upA chemical garden A chemical garden is a set of complex biological-looking structures created by mixing inorganic chemicals. Chemical gardening is an experiment in chemistry usually performed by adding metal salts, such as copper sulfate or cobalt(II) chloride, to an aqueous solution of sodium silicate (otherwise known as waterglass). This results in the growth of plant-like forms in minutes to hours. The chemical garden was first observed and described by Johann Rudolf Glauber in 1646. In its original form, the chemical garden involved the introduction of ferrous chloride (FeCl2) crystals into a solution of potassium silicate (K2SiO3). Process The chemical garden relies on most transition metal silicates being insoluble in water and colored. When a metal salt, such as cobalt chloride, is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Displacement Reaction
A salt metathesis reaction, sometimes called a double displacement reaction, is a chemical process involving the exchange of chemical bond, bonds between two reacting chemical species which results in the creation of products with similar or identical bonding affiliations. This reaction is represented by the general scheme: :AB + CD -> AD + CB The bond between the reacting species can be either Ionic bonding, ionic or Covalent bond, covalent. Classically, these reactions result in the precipitation of one product. In older literature, the term double decomposition is frequently encountered. The term double decomposition is more specifically used when at least one of the substances does not dissolve in the solvent, as the ligand or ion exchange takes place in the solid state of the reactant. For example: :AX(aq) + BY(s) → AY(aq) + BX(s). Types of reactions Counterion exchange Salt metathesis is a common technique for exchanging counterions. The choice of reactants is guid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Sulfate
Zinc sulfate is an inorganic compound. It is used as a dietary supplement to treat zinc deficiency and to prevent the condition in those at high risk. Side effects of excess supplementation may include abdominal pain, vomiting, headache, and tiredness. The most common form includes water of crystallization as the heptahydrate, with the formula . It was historically known as "white vitriol". Zinc sulfate and its hydrates are colourless solids. Uses Medicine In medicine it is used together with oral rehydration therapy (ORT) and an astringent. Manufacturing The hydrates, especially the heptahydrate, are the primary forms used commercially. The main application is as a coagulant in the production of rayon. It is also a precursor to the pigment lithopone. It is also used as an electrolyte for zinc electroplating, as a mordant in dyeing, and as a preservative for skins and leather. Other Zinc sulfate is used to supply zinc in animal feeds, fertilizers, toothpaste, and agricu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Chloride
Calcium chloride is an inorganic compound, a salt with the chemical formula . It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide. Calcium chloride is commonly encountered as a hydrated solid with generic formula , where ''n'' = 0, 1, 2, 4, and 6. These compounds are mainly used for de-icing and dust control. Because the anhydrous salt is hydroscopic and deliquescent, it is used as a desiccant.Robert Kemp, Suzanne E. Keegan "Calcium Chloride" in Ullmann's Encyclopedia of Industrial Chemistry 2000, Wiley-VCH, Weinheim. Uses De-icing and freezing-point depression By depressing the freezing point of water, calcium chloride is used to prevent ice formation and is used to de-ice. This application consumes the greatest amount of calcium chloride. Calcium chloride is relatively harmless to plants and soil. As a deicing agent, it is much more effective at lower temperat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(III) Chloride
Iron(III) chloride is the inorganic compound with the formula . Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The colour depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light they appear purple-red. Structure and properties Anhydrous Anhydrous iron(III) chloride has the structure, with octahedral Fe(III) centres interconnected by two-coordinate chloride ligands. Iron(III) chloride has a relatively low melting point and boils at around 315 °C. The vapor consists of the dimer (like aluminium chloride) which increasingly dissociates into the monomeric (with D3h point group molecular symmetry) at higher temperature, in competition with its reversible decomposition to give iron(II) chloride and chlorine gas. Hydrates In addition to the anhydrous material, ferric chloride forms four hydrates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Sulfate
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula Fe SO4·''x''H2O. These compounds exist most commonly as the heptahydrate (''x'' = 7) but several values for x are known. The hydrated form is used medically to treat iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol (vitriol is an archaic name for sulfate), the blue-green heptahydrate (hydrate with 7 molecules of water) is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex e(H2O)6sup>2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas. It is on the World Health Organization's List of Essential Medicines. In 2020, it was the 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel(II) Sulfate
Nickel(II) sulfate, or just nickel sulfate, usually refers to the inorganic compound with the formula NiSO4(H2O)6. This highly soluble blue green coloured salt is a common source of the Ni2+ ion for electroplating. Approximately 40,000 tonnes were produced in 2005. It is mainly used for electroplating of nickel. In 2005–2006, nickel sulfate was the top allergen in patch tests (19.0%).Zug KA, Warshaw EM, Fowler JF Jr, Maibach HI, Belsito DL, Pratt MD, Sasseville D, Storrs FJ, Taylor JS, Mathias CG, Deleo VA, Rietschel RL, Marks J. Patch-test results of the North American Contact Dermatitis Group 2005–2006. Dermatitis. 2009 May–Jun;20(3):149-60. Structures At least seven sulfate salts of nickel(II) are known. These salts differ in terms of their hydration or crystal habit. The common tetragonal hexahydrate crystallizes from aqueous solution between 30.7 and 53.8 °C. Below these temperatures, a heptahydrate crystallises, and above these temperatures an orthorhombic h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(III) Chloride
Chromium(III) chloride (also called chromic chloride) describes any of several chemical compounds with the formula CrCl3, where can be 0, 5, and 6. The anhydrous compound with the formula CrCl3 is a violet solid. The most common form of the trichloride is the dark green hexahydrate, CrCl3. Chromium chlorides find use as catalysts and as precursors to dyes for wool. Structure Anhydrous chromium(III) chloride adopts the YCl3 structure, with Cr3+ occupying one third of the octahedral interstices in alternating layers of a pseudo-cubic close packed lattice of Cl− ions. The absence of cations in alternate layers leads to weak bonding between adjacent layers. For this reason, crystals of CrCl3 cleave easily along the planes between layers, which results in the flaky (micaceous) appearance of samples of chromium(III) chloride. If pressurized to 9.9 GPa it goes under a phase transition. File:Chromium(III)-chloride-sheet-from-monoclinic-xtal-3D-balls-SF-overlay.png, Space-filling m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Sulfate
Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula . It forms hydrates , where ''n'' can range from 1 to 7. The pentahydrate (''n'' = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate. Older names for the pentahydrate include blue vitriol, bluestone, vitriol of copper,Antoine-François de Fourcroy, tr. by Robert Heron (1796) "Elements of Chemistry, and Natural History: To which is Prefixed the Philosophy of Chemistry". J. Murray and others, Edinburgh. Page 348. and Roman vitriol.Oxford University Press,Roman vitriol, Oxford Living Dictionaries. Accessed on 2016-11-13 It exothermically dissolves in water to give the aquo complex , which has octahedral molecular geometry. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. The centers are interconnected by sulfate anions to form chains. Anhydrous copper sulf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alum
An alum () is a type of chemical compound, usually a hydrated double sulfate salt of aluminium with the general formula , where is a monovalent cation such as potassium or ammonium. By itself, "alum" often refers to potassium alum, with the formula . Other alums are named after the monovalent ion, such as sodium alum and ammonium alum. The name "alum" is also used, more generally, for salts with the same formula and structure, except that aluminium is replaced by another trivalent metal ion like chromium, and/or sulfur is replaced by another chalcogen like selenium. The most common of these analogs is chrome alum . In most industries, the name "alum" (or "papermaker's alum") is used to refer to aluminium sulfate, , which is used for most industrial flocculation (the variable is an integer whose size depends on the amount of water absorbed into the alum). In medicine, "alum" may also refer to aluminium hydroxide gel used as a vaccine adjuvant. History Alum found at ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both " ice" and " rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
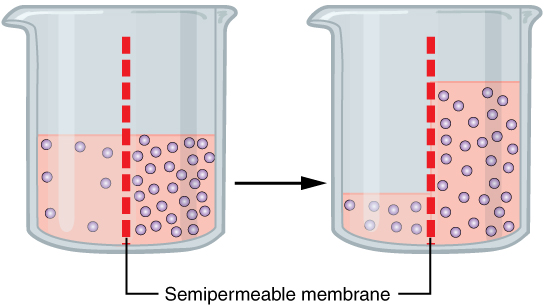
Osmotic
Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of low water potential (region of higher solute concentration), in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane (permeable to the solvent, but not the solute) separating two solutions of different concentrations. Osmosis can be made to do work. Osmotic pressure is defined as the external pressure required to be applied so that there is no net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity. Osmosis is a vital process in biological systems, as biological membranes are semi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)