|
Cerium(IV) Oxide
Cerium(IV) oxide, also known as ceric oxide, ceric dioxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare-earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2. It is an important commercial product and an intermediate in the purification of the element from the ores. The distinctive property of this material is its reversible conversion to a non-stoichiometric oxide. Production Cerium occurs naturally as oxides, always as a mixture with other rare-earth elements. Its principal ores bastnaesite and monazite. After extraction of the metal ions into aqueous base, Ce is separated from that mixture by addition of an oxidant followed by adjustment of the pH. This step exploits the low solubility of CeO2 and the fact that other rare-earth elements resist oxidation.. Cerium(IV) oxide is formed by the calcination of cerium oxalate or cerium hydroxide. Cerium also forms cerium(III) oxide, , which is unstable and will oxidize to ceriu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hygroscopy
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonstoichiometric Compound
In chemistry, non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); most often, in such materials, some small percentage of atoms are missing or too many atoms are packed into an otherwise perfect lattice work. Contrary to earlier definitions, modern understanding of non-stoichiometric compounds view them as homogeneous, and not mixtures of stoichiometric chemical compounds. Since the solids are overall electrically neutral, the defect is compensated by a change in the charge of other atoms in the solid, either by changing their oxidation state, or by replacing them with atoms of different elements with a different charge. Many metal oxides and sulfides have non-stoichiometric examples; for example, stoichiometric iron(II) oxide, which is rare, has the formula , whereas the more common material is nonstoichi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
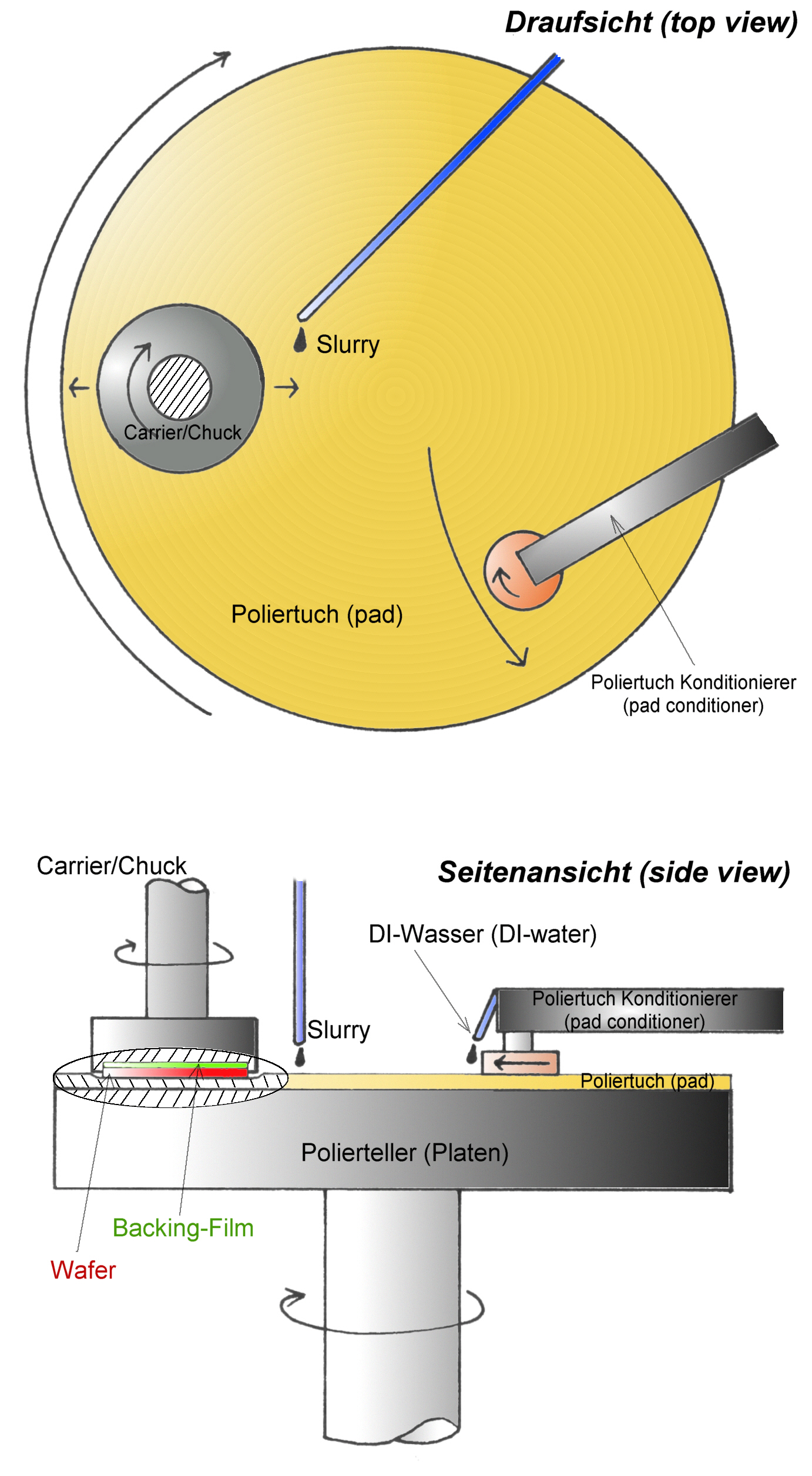
Chemical-mechanical Polishing
Chemical mechanical polishing (CMP) or planarization is a process of smoothing surfaces with the combination of chemical and mechanical forces. It can be thought of as a hybrid of chemical etching and free abrasive polishing. Description The process uses an abrasive and corrosive chemical slurry (commonly a colloid) in conjunction with a polishing pad and retaining ring, typically of a greater diameter than the wafer. The pad and wafer are pressed together by a dynamic polishing head and held in place by a plastic retaining ring. The dynamic polishing head is rotated with different axes of rotation (i.e., not concentric). This removes material and tends to even out any irregular topography, making the wafer flat or planar. This may be necessary to set up the wafer for the formation of additional circuit elements. For example, CMP can bring the entire surface within the depth of field of a photolithography system, or selectively remove material based on its position. Typical depth- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerium Anomaly
The cerium anomaly, in geochemistry, is the phenomenon whereby cerium (Ce) concentration is either depleted or enriched in a rock relative to the other rare-earth elements (REEs). A Ce anomaly is said to be "negative" if Ce is depleted relative to the other REEs and is said to be "positive" if Ce is enriched relative to the other REEs. Cerium oxidation states Cerium is a rare-earth element ( lanthanide) characterized by two different redox states: III and IV. Contrary to other lanthanide elements, which are only trivalent (with the notable exception of Eu2+), Ce3+ can be oxidized by atmospheric oxygen (O2) to Ce4+ under alkaline conditions. The cerium anomaly relates to the decrease in solubility, which accompanies the oxidation of Ce(III) to Ce(IV). Under reducing conditions, Ce3+ is relatively soluble, while under oxidizing conditions CeO2 precipitates. Sediments deposited under oxic or anoxic conditions can preserve on the long term the geochemical signature of Ce3+ or Ce4+ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare Earth Elements
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. Scandium and yttrium are considered rare-earth elements because they tend to occur in the same ore deposits as the lanthanides and exhibit similar chemical properties, but have different electronic and magnetic properties. These metals tarnish slowly in air at room temperature and react slowly with cold water to form hydroxides, liberating hydrogen. They react with steam to form oxides, and at elevated temperature (400°C) ignite spontaneously. These elements and their compounds have no biological function other than in several specialized enzymes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dyrnaesite-(La)
Dyrnaesite-(La) is a rare-earth phosphate mineral with the formula . Dyrnaesite-(La) is related to vitusite-(Ce), another rare-earth phosphate mineral. It comes from lujavrite, a type of alkaline syenite rock, of South Greenland. Dyrnaesite-(La) is one of few known minerals with essential tetravalent cerium, the other two being cerianite-(Ce) Cerianite-(Ce) is a relatively rare oxide mineral, belonging to uraninite group with the formula . It is one of a few currently known minerals containing essential tetravalent cerium, the other examples being stetindite and dyrnaesite-(La). Oc ... and stetindite. References Phosphate minerals Cerium minerals Lanthanide minerals Sodium minerals Orthorhombic minerals Minerals in space group 62 {{phosphate-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerianite-(Ce)
Cerianite-(Ce) is a relatively rare oxide mineral, belonging to uraninite group with the formula . It is one of a few currently known minerals containing essential tetravalent cerium, the other examples being stetindite and dyrnaesite-(La). Occurrence and association Cerianite-(Ce) is associated with alkaline rocks, mostly nepheline syenites. It may be found in carbonatites. Cerianite-(Ce) associates with minerals of the apatite group, bastnäsite-group minerals, calcite, feldspar, "fluocerite", "hydromica", ilmenite, nepheline, magnetite, "törnebohmite" and tremolite. It is the most simple cerium mineral known. Notes on chemistry Beside thorium cerianite-(Ce) may contain trace niobium, yttrium, lanthanum, ytterbium, zirconium and tantalum. Crystal structure For details on crystal structure see cerium(IV) oxide. Both ceria and thoria have a fluorite structure In solid state chemistry, the fluorite structure refers to a common motif for compounds with the formula MX2. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wetting
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with the first one. The degree of wetting (wettability) is determined by a force balance between adhesive and cohesive forces. Wetting is important in the bonding or adherence of two materials. Wetting and the surface forces that control wetting are also responsible for other related effects, including capillary effects. There are two types of wetting: non-reactive wetting and reactive wetting. Wetting deals with three phases of matter: gas, liquid, and solid. It is now a center of attention in nanotechnology and nanoscience studies due to the advent of many nanomaterials in the past two decades (e.g. graphene, Carbon nano tube, carbon nanotube, boron nitride nanomesh). Explanation Adhesive forces between a liquid and solid cause a liqu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polaron
A polaron is a quasiparticle used in condensed matter physics to understand the interactions between electrons and atoms in a solid material. The polaron concept was proposed by Lev Landau in 1933 and Solomon Pekar in 1946 to describe an electron moving in a dielectric crystal where the atoms displace from their equilibrium positions to effectively screen the charge of an electron, known as a phonon cloud. This lowers the electron mobility and increases the electron's effective mass. The general concept of a polaron has been extended to describe other interactions between the electrons and ions in metals that result in a bound state, or a lowering of energy compared to the non-interacting system. Major theoretical work has focused on solving Fröhlich and Holstein Hamiltonians. This is still an active field of research to find exact numerical solutions to the case of one or two electrons in a large crystal lattice, and to study the case of many interacting electrons. Experim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid-oxide Fuel Cell
A solid oxide fuel cell (or SOFC) is an electrochemical conversion device that produces electricity directly from oxidizing a fuel. Fuel cells are characterized by their electrolyte material; the SOFC has a solid oxide or ceramic electrolyte. Advantages of this class of fuel cells include high combined heat and power efficiency, long-term stability, fuel flexibility, low emissions, and relatively low cost. The largest disadvantage is the high operating temperature which results in longer start-up times and mechanical and chemical compatibility issues. Introduction Solid oxide fuel cells are a class of fuel cells characterized by the use of a solid oxide material as the electrolyte. SOFCs use a solid oxide electrolyte to conduct negative oxygen ions from the cathode to the anode. The electrochemical oxidation of the hydrogen, carbon monoxide or other organic intermediates by oxygen ions thus occurs on the anode side. More recently, proton-conducting SOFCs (PC-SOFC) are being deve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Conductivity (solid State)
Ionic conductivity (denoted by ) is a measure of a substance's tendency towards ionic conduction. Ionic conduction is the movement of ions. The phenomenon is observed in solids and solutions. Ionic conduction is one mechanism of current. In crystalline solids In most solids, ions rigidly occupy fixed positions, strongly embraced by neighboring atoms or ions. In some solids, selected ions are highly mobile allowing ionic conduction. The mobility increases with temperature. Materials exhibiting this property are used in batteries. A well-known ion conductive solid is β''-alumina ("BASE"), a form of aluminium oxide that has channels through which sodium cations can hop. When this ceramic is complexed with a mobile ion, such as Na+, it behaves as so-called fast ion conductor. BASE is used as a membrane in several types of molten salt electrochemical cell. In glasses Ion conduction in disordered solids like glasses, polymers, nanocomposites, defective crystals and other di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |