|
Tellurides
The telluride ion is the anion Te2− and its derivatives. It is analogous to the other chalcogenide anions, the lighter O2−, S2−, and Se2−, and the heavier Po2−. In principle, Te2− is formed by the two-e− reduction of tellurium. The redox potential is −1.14 V. :Te(s) + 2 e− ↔ Te2− Although solutions of the telluride dianion have not been reported, soluble salts of bitelluride (TeH−) are known. Organic tellurides ''Tellurides'' also describe a class of organotellurium compounds formally derived from Te2−. An illustrative member is dimethyl telluride, which results from the methylation of telluride salts: :2 CH3I + Na2Te → (CH3)2Te + 2 NaI Dimethyl telluride is formed by the body when tellurium is ingested. Such compounds are often called telluroethers because they are structurally related to ethers with tellurium replacing oxygen, although the length of the C–Te bond is much longer than a C–O bond. C–Te–C angles tend to be closer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Telluride
Hydrogen telluride is the inorganic compound with the formula H2 Te. A hydrogen chalcogenide and the simplest hydride of tellurium, it is a colorless gas. Although unstable in ambient air, the gas can exist long enough to be readily detected by the odour of rotting garlic at extremely low concentrations; or by the revolting odour of rotting leeks at somewhat higher concentrations. Most compounds with Te–H bonds (tellurols) are unstable with respect to loss of H2. H2Te is chemically and structurally similar to hydrogen selenide, both are acidic. The H–Te–H angle is about 90°. Volatile tellurium compounds often have unpleasant odours, reminiscent of decayed leeks or garlic.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Synthesis Electrolytic methods have been developed.F. Fehér, "Hydrogen Telluride" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
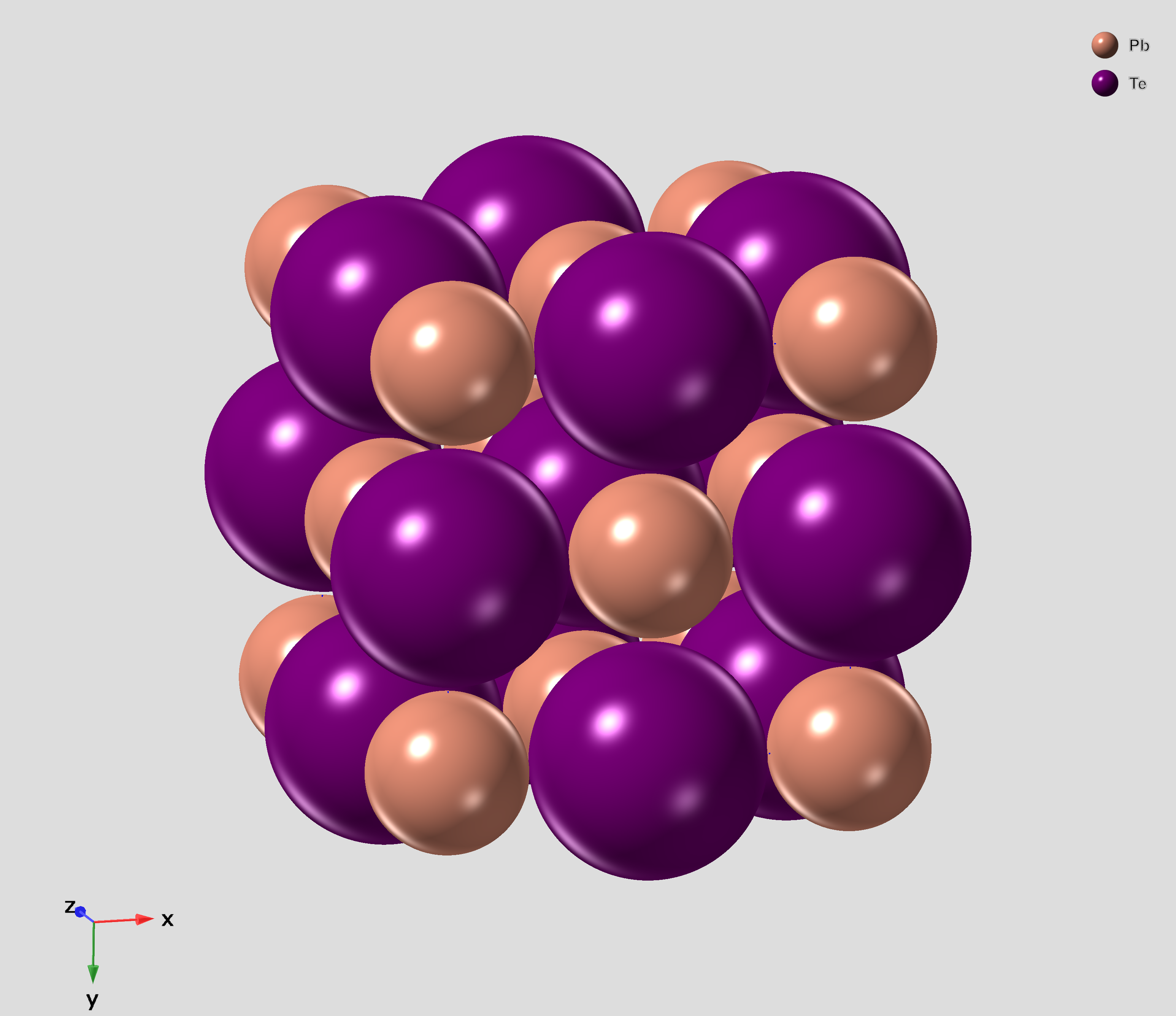
Lead Telluride
Lead telluride is a compound of lead (element), lead and tellurium (PbTe). It crystallizes in the NaCl crystal structure with Pb atoms occupying the cation and Te forming the anionic lattice. It is a narrow gap semiconductor with a band gap of 0.32 eV. It occurs naturally as the mineral altaite. Properties * Dielectric constant ~1000. * Electron Effective mass (solid-state physics), Effective mass ~ 0.01Electron rest mass, ''m''e * Hole mobility, μp = 600 cm2 V−1 s−1 (0 K); 4000 cm2 V−1 s−1 (300 K) * Seebeck coefficient: ~326 μV/K (undoped, at 300K), ~200 μV/K (Ag-doped) Applications PbTe has proven to be a very important intermediate Thermoelectric materials, thermoelectric material. The performance of thermoelectric materials can be evaluated by the figure of merit, ZT=S^2\sigma T/\kappa, in which S is the Seebeck coefficient, \sigma is the Electrical resistivity and conductivity, electrical conducti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuth Telluride
Bismuth telluride () is a gray powder that is a compound of bismuth and tellurium also known as bismuth(III) telluride. It is a semiconductor, which, when alloyed with antimony or selenium, is an efficient thermoelectric material for refrigeration or portable power generation. is a topological insulator, and thus exhibits thickness-dependent physical properties. Properties as a thermoelectric material Bismuth telluride is a narrow-gap layered semiconductor with a trigonal unit cell. The valence and conduction band structure can be described as a many-ellipsoidal model with 6 constant-energy ellipsoids that are centered on the reflection planes. cleaves easily along the trigonal axis due to Van der Waals bonding between neighboring tellurium atoms. Due to this, bismuth-telluride-based materials used for power generation or cooling applications must be polycrystalline. Furthermore, the Seebeck coefficient of bulk becomes compensated around room temperature, forcing the material ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tellurium
Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in its native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth. Tellurium-bearing compounds were first discovered in 1782 in a gold mine in Kleinschlatten, Transylvania (now Zlatna, Romania) by Austrian mineralogist Franz-Joseph Müller von Reichenstein, although it was Martin Heinrich Klaproth who named the new element in 1798 after the Latin 'earth'. Gold telluride minerals are the most notable natural gold compounds. However, they are not a commercially signif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polytelluride
In chemistry, a polytelluride usually refers to anions of the formula (Te''n'')2−. Many main group and transition metals form complexes with polytelluride anions. Preparation Conceptually, polytellurides are derived from polytelluranes H2Ten, but such neutral species are not known (even H2Te is labile). Instead, analogous to the preparation of many Zintl ions, polytellurides are produced by reduction of elemental Te with alkali metals. Such reactions can be conducted by heating a mixture of the solids or by dissolving Te metal in amine solvents of alkali metals. Once generated, these alkali metal polytellurides can be converted to lipophilic salts by treatment cryptand ligands or by ion exchange with quat salts. Structures Salts of polytellurides have often been characterized by X-ray crystallography. Polytelluride salts generally feature open chains, which adopt a zig-zag conformation. In some cases, cyclic structures are observed as in Li2Te7, which features a square-pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Telluride Mineral
A telluride mineral is a mineral In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Mi ... that has the telluride anion as a main component. Tellurides are similar to sulfides and are grouped with them in both the Dana and Strunz mineral classification systems.http://webmineral.com/strunz/II.shtml Webmineral Strunz Examples include: * altaite * calaverite * coloradoite * empressite * hessite * kostovite * krennerite * melonite * merenskyite * petzite * rickardite * stützite * sylvanite * tellurobismuthite * temagamite * tetradymite * vulcanite See also * Cripple Creek & Victor Gold Mine References * {{Mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Telluride
Dimethyl telluride is an organotelluride compound, formula ( CH3)2 Te, also known by the abbreviation DMTe. This was the first material used to grow epitaxial cadmium telluride and mercury cadmium telluride using metalorganic vapour phase epitaxy. Dimethyl telluride as a product of microbial metabolism was first discovered in 1939. It is produced by some fungi and bacteria ('' Penicillium brevicaule'', ''P. chrysogenum'', and '' P. notatum'' and the bacterium ''Pseudomonas fluorescens''). The toxicity of DMTe is unclear. It is produced by the body when tellurium or one of its compounds are ingested. It is noticeable by the garlic smelling breath it gives those exposed, similar to the effect of DMSO. Tellurium is known to be toxic Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Telluride Photovoltaics
Cadmium telluride (CdTe) photovoltaics is a photovoltaic (PV) technology based on the use of cadmium telluride in a thin semiconductor material, semiconductor layer designed to absorb and convert sunlight into electricity. Cadmium telluride PV is the only thin film solar cell, thin film technology with lower costs than conventional solar cells made of crystalline silicon in multi-kilowatt systems.K. Zweibel, J. Mason, V. Fthenakis,A Solar Grand Plan, ''Scientific American'', Jan 2008. CdTe PV is the cheapest example of PV technologies and prices are about 16¢/kWh with US Southwest sunlight. On a lifecycle basis, CdTe PV has the smallest carbon footprint, lowest water use and shortest Crystalline silicon#Energy payback time, energy payback time of any current photovoltaic technology. CdTe's energy payback time of less than a year allows for faster carbon reductions without short-term energy deficits. The toxicity of cadmium is an environmental concern during production and whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aurostibite
Aurostibite is an isometric gold antimonide mineral which is a member of the pyrite group. Aurostibite was discovered in 1952 and can be found in hydrothermal gold-quartz veins, in sulfur-deficient environments that contain other antimony minerals. The mineral can be found in Yellowknife in the Northwest Territories of Canada, and the Timiskaming District in Ontario, Canada. Antimonides are rare and are normally placed in the sulfide class by mineralogists. See also * List of minerals This is a list of minerals which have Wikipedia articles. Minerals are distinguished by various chemical and physical properties. Differences in chemical composition and crystal structure distinguish the various ''species''. Within a mineral speci ... References Gold minerals Antimonide minerals Pyrite group Cubic minerals Minerals in space group 205 Minerals described in 1952 {{mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sylvanite
Sylvanite or silver gold telluride, chemical formula , is the most common telluride of gold. Properties The gold:silver ratio varies from 3:1 to 1:1. It is a metallic mineral with a color that ranges from a steely gray to almost white. It is closely related to calaverite, which is more purely gold telluride with 3% silver. Sylvanite crystallizes in the monoclinic 2/m system. Crystals are rare and it is usually bladed or granular. It is very soft with a hardness of 1.5–2. It has a high relative density of 8–8.2. Sylvanite is photosensitive and can accumulate a dark tarnish if it is exposed to bright light for too long. Occurrence Sylvanite is found in Transylvania, from which its name is partially derived. It is also found and mined in Australia in the East Kalgoorlie district. In Canada it is found in the Kirkland Lake Gold District, Ontario and the Rouyn District, Quebec. In the United States it occurs in California and in Colorado where it was mined as part of the Cri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Krennerite
Krennerite is an orthorhombic gold telluride mineral which can contain variable amounts of silver in the structure. The formula is AuTe2, but specimen with gold substituted by up to 24% with silver have been found ( u0.77Ag0.24e2). Both of the chemically similar gold-silver tellurides, calaverite and sylvanite, are in the monoclinic crystal system, whereas krennerite is orthorhombic. The color varies from silver-white to brass-yellow. It has a specific gravity of 8.62 and a hardness of 2.5. It occurs in high temperature, hydrothermal environments. Krennerite was discovered in 1878 near the village of Săcărâmb, Romania, and first described by the Hungarian mineralogist Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical mineralogy, optical) properties of minerals and mineralized artifact (archaeology), artifacts. Specific s ... Joseph Krenner (1839–1920). See also * List of mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |