|
Scandium
Scandium is a chemical element; it has Symbol (chemistry), symbol Sc and atomic number 21. It is a silvery-white metallic d-block, d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the lanthanides. It was discovered in 1879 by spectral analysis of the minerals euxenite and gadolinite from Scandinavia. Scandium is present in most of the deposits of rare-earth and uranium compounds, but it is extracted from these ores in only a few mines worldwide. Because of the low availability and difficulties in the preparation of metallic scandium, which was first done in 1937, applications for scandium were not developed until the 1970s, when the positive effects of scandium on aluminium alloys were discovered. Its use in such alloys remains its only major application. The global trade of scandium oxide is 15–20 tonnes per year. The properties of scandium compounds are intermediate between those of aluminium and yttrium. A diagonal rela ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scandium Oxide
Scandium(III) oxide or scandia is a inorganic compound with formula scandium, Sc2oxygen, O3. It is one of several oxides of rare earth elements with a high melting point. It is used in the preparation of other scandium compounds as well as in high-temperature systems (for its resistance to heat and thermal shock), electronic ceramics, and glass composition (as a helper material). Structure and physical properties Scandium(III) oxide adopts a Cubic crystal system, cubic crystal structure (point group: tetrahedral (Th), space group: Ia) containing 6-coordinate metal centres. Powder diffraction analysis shows Sc−O bond distances of 2.159–2.071 Å. Scandium oxide is an insulator with a band gap of 6.0 eV. Production Scandium oxide is the primary form of refined scandium produced by the mining industry. Scandium-rich ores, such as thortveitite (Sc,Y)2(Si2O7) and kolbeckite ScPO4·2H2O are rare, however trace amounts of scandium are present in many other minerals. Scan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare-earth Element
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set of 17 nearly indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. The term "rare-earth" is a misnomer because they are not actually scarce, but historically it took a long time to isolate these elements. They are relatively plentiful in the entire Earth's crust (cerium being the 25th-most-abundant element at 68 parts per million, more abundant than copper), but in practice they are spread thinly as trace impurities, so to obtain rare earths at usable purity requires processing enormous amounts of raw ore at great expense; thus the name "rare" earths. Scandium and yttrium are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Alloy
An aluminium alloy ( UK/IUPAC) or aluminum alloy ( NA; see spelling differences) is an alloy in which aluminium (Al) is the predominant metal. The typical alloying elements are copper, magnesium, manganese, silicon, tin, nickel and zinc. There are two principal classifications, namely casting alloys and wrought alloys, both of which are further subdivided into the categories heat-treatable and non-heat-treatable. About 85% of aluminium is used for wrought products, for example rolled plate, foils and extrusions. Cast aluminium alloys yield cost-effective products due to their low melting points, although they generally have lower tensile strengths than wrought alloys. The most important cast aluminium alloy system is Al–Si, where the high levels of silicon (4–13%) contribute to give good casting characteristics. Aluminium alloys are widely used in engineering structures and components where light weight or corrosion resistance is required.I. J. Polmear, ''Light Alloys'', A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scandium-44
Scandium-44 (44Sc) is a radioactive isotope of scandium that decays by positron emission to stable 44Ca with a half-life of 4.042 hours. 44Sc can be obtained as a daughter radionuclide of long-lived 44Ti (t1/2 60.4 a) from 44Ti /44Sc generator or can be produced by nuclear reaction 44Ca ( p, n)44Sc in small cyclotrons. This isotope is of potential interest for clinical PET imaging. Applications Titanium-44 generation 44Ti with its long half-life of ca. 60 years provides a cyclotron-independent source of 44Sc for several decades. 44Ti is obtained in the nuclear reaction 45Sc(p,2n)44Ti and it transforms directly into the ground state of 44Sc via electron capture, emitting low energetic photons at 67.9 keV and 78.3 keV. The 44Ti /44Sc generator represents a secular equilibrium system with a half-life ratio between parent and daughter of ca. 130 000. Consequently, 50% of saturation activity is generated every 3.97 hours, and identical 44Sc batch activities very close to saturat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium
Yttrium is a chemical element; it has Symbol (chemistry), symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals and is never found in nature as a free element. 89Y is the only stable isotope and the only isotope found in the Crust (geology), Earth's crust. The most important present-day use of yttrium is as a component of phosphors, especially those used in LEDs. Historically, it was once widely used in the red phosphors in television set cathode ray tube displays. Yttrium is also used in the production of electrodes, electrolytes, electronic filters, lasers, superconductors, various medical applications, and Trace element, tracing various materials to enhance their properties. Yttrium has no known Biology, biological role. Exposure to yttrium compounds can cause Respiratory ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (element 71) is also sometimes considered a lanthanide, despite being a d-block element and a transition metal. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. Lutetium is a d-block element (thus also a transition metal), and on this basis its inclusion has been questioned; however, like its congeners scandium and yttrium in group 3, it behaves similarly to the other 14. The term rare-earth element or rare-earth metal is often used to include the stable group 3 elements Sc, Y, and Lu in addition to the 4f elements. All lanthanide elements form trivalent cations, Ln3+, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gadolinite
Gadolinite, sometimes known as ytterbite, is a silicate mineral consisting principally of the silicates of cerium, lanthanum, neodymium, yttrium, beryllium, and iron with the formula . It is called gadolinite-(Ce) or gadolinite-(Y), depending on the prominent composing element (Y if yttrium predominates, and Ce if cerium). It may contain 35.5% yttria sub-group rare earths, 2.2% ceria earths, as much as to 11.6% BeO, and traces of thorium. It is found in Sweden, Norway, and the US (Texas and Colorado). Characteristics Gadolinite is fairly rare and typically occurs as well-formed crystals. It is nearly black in color and has a vitreous luster. The hardness is between 6.5 and 7 on the Mohs scale, and the specific gravity is between 4.0 and 4.7. It fractures in a conchoidal pattern and streaks grayish-green. It was also thought to exhibit pyrognomic properties, as it can emit visible light when heated to relatively low temperatures, but the scientific consensus is that this is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symbol (chemistry)
Chemical symbols are the abbreviations used in chemistry, mainly for chemical elements; but also for functional groups, chemical compounds, and other entities. Element symbols for chemical elements, also known as atomic symbols, normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. History Earlier symbols for chemical elements stem from classical Latin and Greek words. For some elements, this is because the material was known in ancient times, while for others, the name is a more recent invention. For example, Pb is the symbol for lead (''plumbum'' in Latin); Hg is the symbol for mercury (''hydrargyrum'' in Greek); and He is the symbol for helium (a Neo-Latin name) because helium was not known in ancient Roman times. Some symbols come from other sources, like W for tungsten (''Wolfram'' in German) which was not known in Roman times. A three-letter temporary symbol may be assigned to a newly synthesized (or not yet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
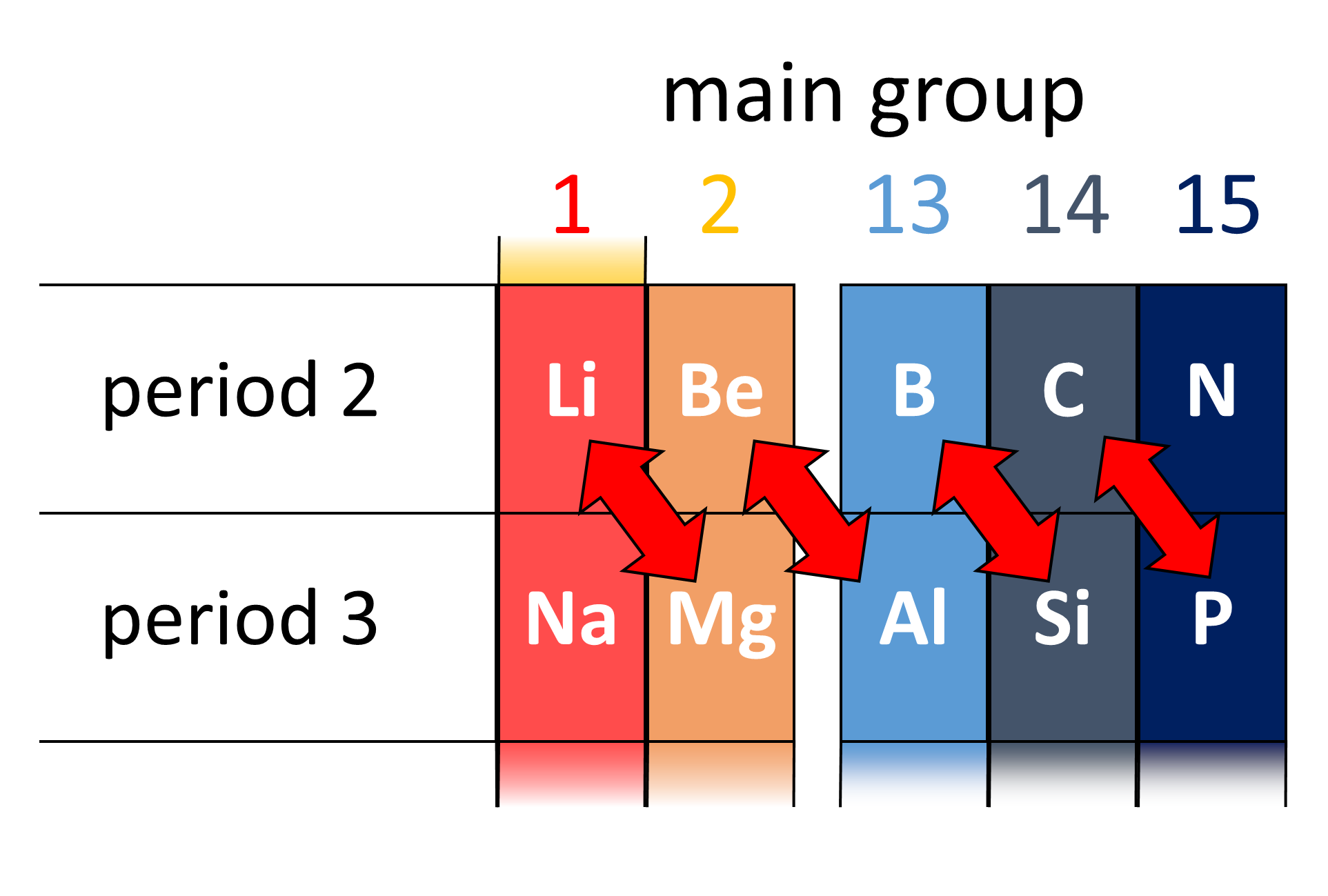
Diagonal Relationship
In chemistry, a diagonal relationship is said to exist between certain pairs of diagonally adjacent elements in the second and third periods (first 20 elements) of the periodic table. These pairs (lithium (Li) and magnesium (Mg), beryllium (Be) and aluminium (Al), boron (B) and silicon (Si), etc.) exhibit similar properties; for example, boron and silicon are both semiconductors, forming halides that are hydrolysed in water and have acidic oxides. Further diagonal similarities have also been suggested for carbon-phosphorus and nitrogen-sulfur, along with extending the Li-Mg and Be-Al relationships down into the transition elements (such as scandium). The organization of elements on the periodic table into horizontal rows and vertical columns makes certain relationships more apparent ( periodic law). Moving rightward and descending the periodic table have opposite effects on atomic radii of isolated atoms. Moving rightward across the period decreases the atomic radii of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has a great affinity towards oxygen, passivation (chemistry), forming a protective layer of aluminium oxide, oxide on the surface when exposed to air. It visually resembles silver, both in its color and in its great ability to reflect light. It is soft, magnetism, nonmagnetic, and ductility, ductile. It has one stable isotope, 27Al, which is highly abundant, making aluminium the abundance of the chemical elements, 12th-most abundant element in the universe. The radioactive decay, radioactivity of aluminium-26, 26Al leads to it being used in radiometric dating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Passivation (chemistry)
In physical chemistry and engineering, passivation is coating a material so that it becomes "passive", that is, less readily affected or corroded by the environment. Passivation involves creation of an outer layer of shield material that is applied as a microcoating, created by chemical reaction with the base material, or allowed to build by spontaneous oxidation in the air. As a technique, passivation is the use of a light coat of a protective material, such as metal oxide, to create a shield against corrosion. Passivation of silicon is used during fabrication of microelectronic devices. Undesired passivation of electrodes, called "fouling", increases the circuit resistance so it interferes with some electrochemical applications such as electrocoagulation for wastewater treatment, amperometric chemical sensing, and electrochemical synthesis. When exposed to air, many metals naturally form a hard, relatively inert surface layer, usually an oxide (termed the "native oxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its atomic nucleus, nucleus. Atoms of the same element can have different numbers of neutrons in their nuclei, known as isotopes of the element. Two or more atoms can combine to form molecules. Some elements form Homonuclear molecule, molecules of atoms of said element only: e.g. atoms of hydrogen (H) form Diatomic molecule, diatomic molecules (H). Chemical compounds are substances made of atoms of different elements; they can have molecular or non-molecular structure. Mixtures are materials containing different chemical substances; that means (in case of molecular substances) that they contain different types of molecules. Atoms of one element can be transformed into atoms of a different element in nuclear reactions, which change an atom's at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |