|
Lanthanum
Lanthanum is a chemical element; it has symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table, of which lanthanum is the first and the prototype. Lanthanum is traditionally counted among the rare earth elements. Like most other rare earth elements, its usual oxidation state is +3, although some compounds are known with an oxidation state of +2. Lanthanum has no biological role in humans but is used by some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity. Lanthanum usually occurs together with cerium and the other rare earth elements. Lanthanum was first found by the Swedish chemist Carl Gustaf Mosander in 1839 as an impurity in cerium nitrate – hence the name ''lanthanum'', from the ancient Greek (), meaning 'to lie hidden'. Although ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (element 71) is also sometimes considered a lanthanide, despite being a d-block element and a transition metal. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. Lutetium is a d-block element (thus also a transition metal), and on this basis its inclusion has been questioned; however, like its congeners scandium and yttrium in group 3, it behaves similarly to the other 14. The term rare-earth element or rare-earth metal is often used to include the stable group 3 elements Sc, Y, and Lu in addition to the 4f elements. All lanthanide elements form trivalent cations, Ln3+, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum Carbonate
Lanthanum carbonate, La( C O3)3, is the salt formed by lanthanum(III) cations and carbonate anions. It is an ore of lanthanum metal ( bastnäsite), along with monazite. Chemistry Lanthanum carbonate is used as a starting material in lanthanum chemistry, particularly in forming mixed oxides, for example * for production of lanthanum strontium manganite, primarily for solid oxide fuel cell applications; * for production of certain high-temperature superconductors, such as LaSrCuO. Medical uses Lanthanum carbonate is used in medicine as a phosphate binder. As a medication it is sold under the trade name Fosrenol by the pharmaceutical company Shire Pharmaceuticals. Due to its large size (1000 mg tablet is 2.2 cm in diameter), it may be possible to choke on the tablet if it is not chewed. It is prescribed for the treatment of hyperphosphatemia, primarily in patients with chronic kidney disease. It is taken with meals and binds to dietary phosphate, preventing phosp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate Binder
Phosphate binders are medications used to reduce the absorption of dietary phosphate; they are taken along with meals and snacks. They are frequently used in people with chronic kidney failure (CKF), who are less able to excrete phosphate, resulting in an elevated serum phosphate. Mechanism of action These agents work by binding to phosphate in the GI tract, thereby making it unavailable to the body for absorption. Hence, these drugs are usually taken with meals to bind any phosphate that may be present in the ingested food. Phosphate binders may be simple molecular entities (such as magnesium, aluminium, calcium, or lanthanum salts) that react with phosphate and form an insoluble compound. Calcium carbonate Calcium-based phosphate binders, such as calcium carbonate, directly decrease phosphate levels by creating insoluble calcium–phosphate complexes which gets eliminated in the feces. Lanthanum carbonate Non-calcium-based phosphate binders, including lanthanum carbonate, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the cerium-dominant member of the group. It occurs usually in small isolated crystals. It has a hardness of 5.0 to 5.5 on the Mohs scale of mineral hardness and is relatively dense, about 4.6 to 5.7 g/cm3. There are five different most common species of monazite, depending on the relative amounts of the rare earth elements in the mineral: * monazite-(Ce), (the most common member), * monazite-(La), , * monazite-(Nd), , * monazite-(Sm), , * monazite-(Pr), . The elements in parentheses are listed in the order of their relative proportion within the mineral: lanthanum is the most common rare-earth element in monazite-(La), and so forth. Silica () is present in trace amounts, as well as small amounts of uranium and thorium. Due to the alpha decay o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
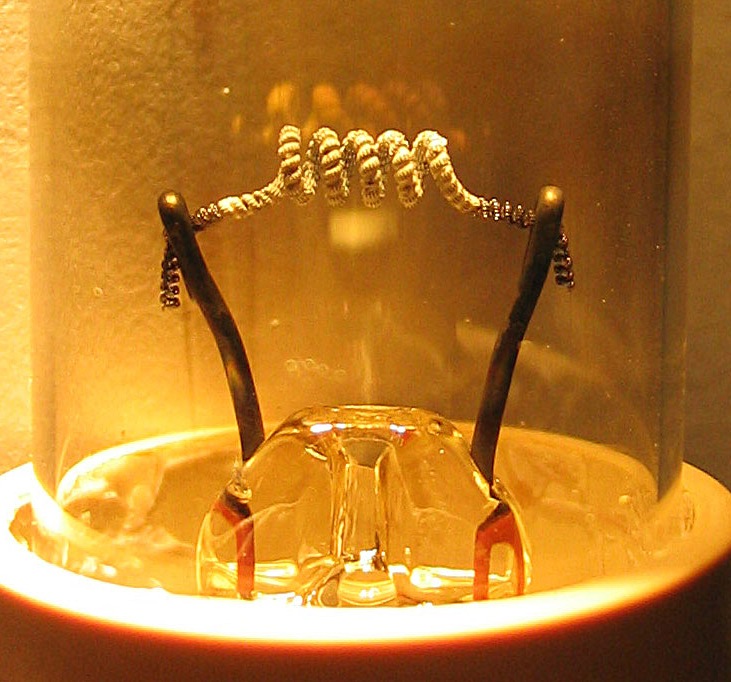
Hot Cathode
In vacuum tubes and gas-filled tubes, a hot cathode or thermionic cathode is a cathode electrode which is heated to make it emit electrons due to thermionic emission. This is in contrast to a cold cathode, which does not have a heating element. The heating element is usually an electrical filament heated by a separate electric current passing through it. Hot cathodes typically achieve much higher power density than cold cathodes, emitting significantly more electrons from the same surface area. Cold cathodes rely on field electron emission or secondary electron emission from positive ion bombardment, and do not require heating. There are two types of hot cathode. In a ''directly heated cathode'', the filament is the cathode and emits the electrons. In an ''indirectly heated cathode'', the filament or ''heater'' heats a separate metal cathode electrode which emits the electrons. From the 1920s to the 1960s, a wide variety of electronic devices used hot-cathode vacuum tubes. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carl Gustaf Mosander
Carl Gustaf Mosander (10 September 1797 – 15 October 1858) was a Swedish chemist. He discovered the rare earth elements lanthanum, erbium and terbium. Early life and education Born in Kalmar, Mosander attended school there until he moved to Stockholm with his mother in 1809. In Stockholm, he became an apprentice at the ''Ugglan'' pharmacy. He took his pharmacy examination in 1817, but had an interest in medicine and joined the Karolinska Institute in 1820. He passed his medical examination in 1825. He worked in the laboratory of Jöns Jakob Berzelius and became a close friend of fellow student Friedrich Wöhler. Career In 1832 Jöns Jakob Berzelius retired in favor of Mosander, his student, who succeeded him as professor of chemistry and pharmacy in the Karolinska Institute. From 1845 Mosander was also a professor at and inspector for the Pharmaceutical Institute. Mosander was an assistant curator of the mineralogical collections of the Swedish Museum of Natural Hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare Earth Element
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set of 17 nearly indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. The term "rare-earth" is a misnomer because they are not actually scarce, but historically it took a long time to isolate these elements. They are relatively plentiful in the entire Earth's crust ( cerium being the 25th-most-abundant element at 68 parts per million, more abundant than copper), but in practice they are spread thinly as trace impurities, so to obtain rare earths at usable purity requires processing enormous amounts of raw ore at great expense; thus the name "rare" earths. Scandium and yttrium a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is , meaning that the 1s, 2s, and 2p subshells are occupied by two, two, and six electrons, respectively. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by the nuclei and all the other electrons. Mathematically, configurations are described by Slater determinants or configuration state functions. According to the laws of quantum mechanics, a level of energy is associated with each electron configuration. In certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon. Knowledge of the electron configuration of different atoms is useful in understanding the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodic Table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which states that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. The table is divided into four roughly rectangular areas called blocks. Elements in the same group tend to show similar chemical characteristics. Vertical, horizontal and diagonal trends characterize the periodic table. Metallic character increases going down a group and from right to left across a period. Nonmetallic character increases going from the bottom left of the periodic table to the top right. The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869; he formulated the periodic law as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symbol (chemistry)
Chemical symbols are the abbreviations used in chemistry, mainly for chemical elements; but also for functional groups, chemical compounds, and other entities. Element symbols for chemical elements, also known as atomic symbols, normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. History Earlier symbols for chemical elements stem from classical Latin and Greek words. For some elements, this is because the material was known in ancient times, while for others, the name is a more recent invention. For example, Pb is the symbol for lead (''plumbum'' in Latin); Hg is the symbol for mercury (''hydrargyrum'' in Greek); and He is the symbol for helium (a Neo-Latin name) because helium was not known in ancient Roman times. Some symbols come from other sources, like W for tungsten (''Wolfram'' in German) which was not known in Roman times. A three-letter temporary symbol may be assigned to a newly synthesized (or not yet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Tungsten Arc Welding
Gas tungsten arc welding (GTAW, also known as tungsten inert gas welding or TIG, tungsten argon gas welding or TAG, and heliarc welding when helium is used) is an arc welding process that uses a non-consumable tungsten electrode to produce the weld. The weld area and electrode are protected from oxidation or other atmospheric contamination by an inert shielding gas (argon or helium). A filler metal is normally used, though some welds, known as ' autogenous welds', or ' fusion welds' do not require it. A constant-current welding power supply produces electrical energy, which is conducted across the arc through a column of highly ionized gas and metal vapors known as a plasma. The process grants the operator greater control over the weld than competing processes such as shielded metal arc welding and gas metal arc welding, allowing stronger, higher-quality welds. However, TIG welding is comparatively more complex and difficult to master, and furthermore, it is significantly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scintillator
A scintillator ( ) is a material that exhibits scintillation, the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate (i.e. re-emit the absorbed energy in the form of light). Sometimes, the excited state is metastable, so the relaxation back down from the excited state to lower states is delayed (necessitating anywhere from a few nanoseconds to hours depending on the material). The process then corresponds to one of two phenomena: delayed fluorescence or phosphorescence. The correspondence depends on the type of transition and hence the wavelength of the emitted optical photon. Principle of operation A scintillation detector or scintillation counter is obtained when a scintillator is coupled to an electronic light sensor such as a photomultiplier tube (PMT), photodiode, or silicon photomultiplier. PMTs absorb the light emitted by the scintillator and re-emit it in the form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |