|
Energy Conversion
Energy transformation, also known as energy conversion, is the process of changing energy from one form to another. In physics, energy is a quantity that provides the capacity to perform Work (physics), work (e.g. lifting an object) or provides heat. In addition to being converted, according to the law of conservation of energy, energy is transferable to a different location or object or living being, but it cannot be created or destroyed. Limitations in the conversion of thermal energy Conversions to thermal energy from other forms of energy may occur with 100% efficiency. Conversion among non-thermal forms of energy may occur with fairly high efficiency, though there is always some energy dissipated thermally due to friction and similar processes. Sometimes the efficiency is close to 100%, such as when potential energy is converted to kinetic energy as an object falls in a vacuum. This also applies to the opposite case; for example, an object in an Elliptic orbit, elliptical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jupiter
Jupiter is the fifth planet from the Sun and the List of Solar System objects by size, largest in the Solar System. It is a gas giant with a Jupiter mass, mass more than 2.5 times that of all the other planets in the Solar System combined and slightly less than one-thousandth the mass of the Sun. Its diameter is 11 times that of Earth and a tenth that of the Sun. Jupiter orbits the Sun at a distance of , with an orbital period of . It is the List of brightest natural objects in the sky, third-brightest natural object in the Earth's night sky, after the Moon and Venus, and has been observed since prehistoric times. Its name derives from that of Jupiter (god), Jupiter, the chief deity of ancient Roman religion. Jupiter was the first of the Sun's planets to form, and its inward migration during the primordial phase of the Solar System affected much of the formation history of the other planets. Jupiter's atmosphere consists of 76% hydrogen and 24% helium by mass, with a denser ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
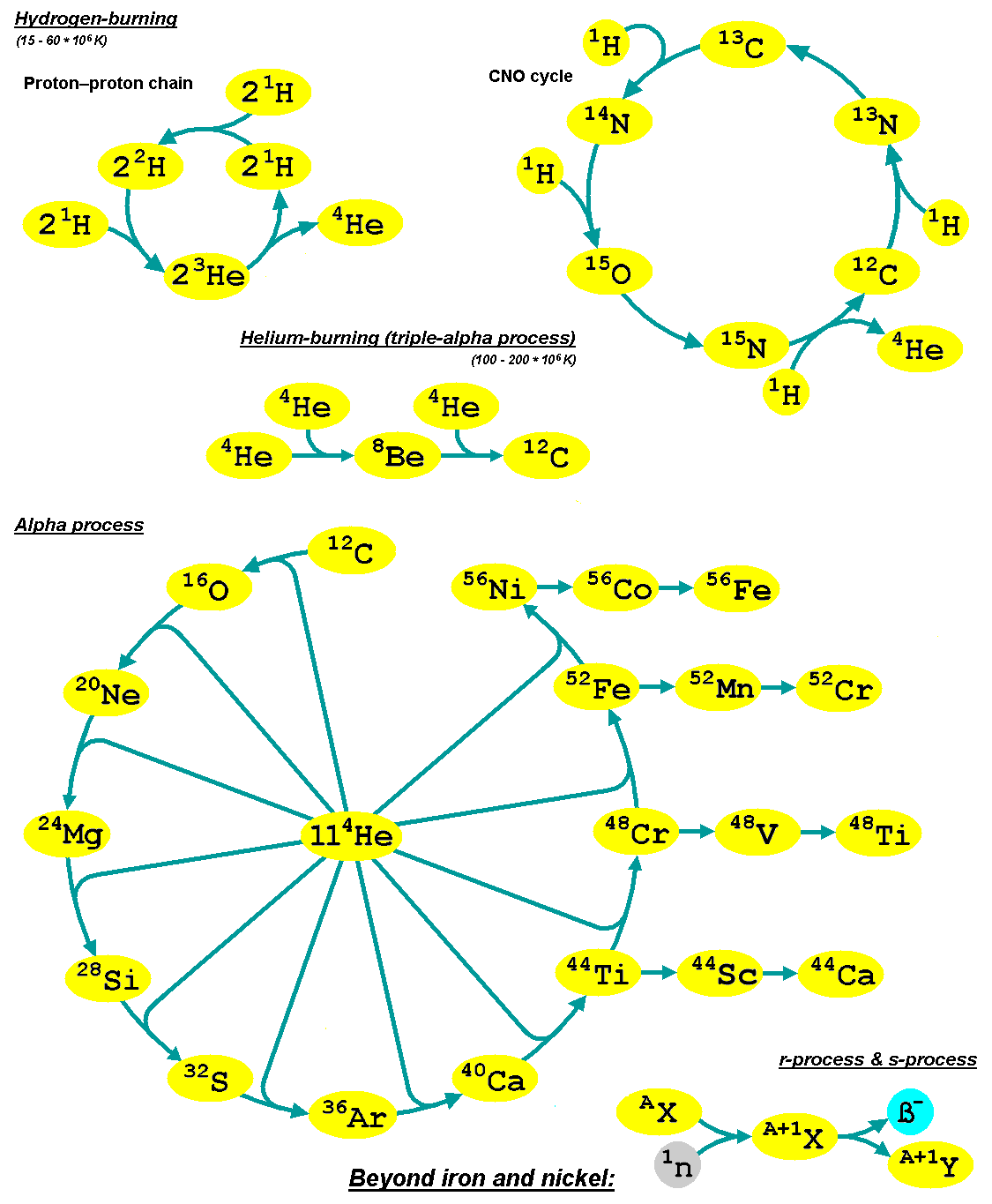
Nuclear Fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the release or absorption (electromagnetic radiation), absorption of energy. This difference in mass arises as a result of the difference in nuclear binding energy between the atomic nuclei before and after the fusion reaction. Nuclear fusion is the process that powers all active stars, via many Stellar nucleosynthesis, reaction pathways. Fusion processes require an extremely large Lawson criterion, triple product of temperature, density, and confinement time. These conditions occur only in Stellar core, stellar cores, advanced Nuclear weapon design, nuclear weapons, and are approached in List of fusion experiments, fusion power experiments. A nuclear fusion process that produces atomic nuclei lighter than nickel-62 is generally exothermic, due t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactive decay. Nuclear fission was discovered by chemists Otto Hahn and Fritz Strassmann and physicists Lise Meitner and Otto Robert Frisch. Hahn and Strassmann proved that a fission reaction had taken place on 19 December 1938, and Meitner and her nephew Frisch explained it theoretically in January 1939. Frisch named the process "fission" by analogy with biological fission of living cells. In their second publication on nuclear fission in February 1939, Hahn and Strassmann predicted the existence and liberation of additional neutrons during the fission process, opening up the possibility of a nuclear chain reaction. For heavy nuclides, it is an exothermic reaction which can release large amounts of energy both as electromagnetic radiat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive Decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered ''radioactive''. Three of the most common types of decay are Alpha decay, alpha, Beta decay, beta, and Gamma ray, gamma decay. The weak force is the Fundamental interactions, mechanism that is responsible for beta decay, while the other two are governed by the electromagnetic force, electromagnetic and nuclear forces. Radioactive decay is a randomness, random process at the level of single atoms. According to quantum mechanics, quantum theory, it is impossible to predict when a particular atom will decay, regardless of how long the atom has existed. However, for a significant number of identical atoms, the overall decay rate can be expressed as a decay constant or as a half-life. The half-lives of radioactive atoms have a huge range: f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar System" and "solar system" structures in theinaming guidelines document. The name is commonly rendered in lower case ('solar system'), as, for example, in the ''Oxford English Dictionary'' an''Merriam-Webster's 11th Collegiate Dictionary''. is the gravitationally bound Planetary system, system of the Sun and the objects that orbit it. It Formation and evolution of the Solar System, formed about 4.6 billion years ago when a dense region of a molecular cloud collapsed, forming the Sun and a protoplanetary disc. The Sun is a typical star that maintains a hydrostatic equilibrium, balanced equilibrium by the thermonuclear fusion, fusion of hydrogen into helium at its stellar core, core, releasing this energy from its outer photosphere. As ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type II Supernova
A Type II supernova or SNII (plural: ''supernovae'') results from the rapid collapse and violent explosion of a massive star. A star must have at least eight times, but no more than 40 to 50 times, the mass of the Sun () to undergo this type of explosion. Type II supernovae are distinguished from other types of supernovae by the presence of hydrogen in their spectra. They are usually observed in the spiral arms of galaxies and in H II regions, but not in elliptical galaxies; those are generally composed of older, low-mass stars, with few of the young, very massive stars necessary to cause a supernova. Stars generate energy by the nuclear fusion of elements. Unlike the Sun, massive stars possess the mass needed to fuse elements that have an atomic mass greater than hydrogen and helium, albeit at increasingly higher temperatures and pressures, causing correspondingly shorter stellar life spans. The degeneracy pressure of electrons and the energy generated by th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in a process called Big Bang nucleosynthesis. After about 20 minutes, the universe had expanded and cooled to a point at which these high-energy collisions among nucleons ended, so only the fastest and simplest reactions occurred, leaving our universe containing hydrogen and helium. The rest is traces of other elements such as lithium and the hydrogen isotope deuterium. Nucleosynthesis in stars and their explosions later produced the variety of elements and isotopes that we have today, in a process called cosmic chemical evolution. The amounts of total mass in elements heavier than hydrogen and helium (called 'metals' by astrophysicists) remains small (few percent), so that the universe still has approximately the same composition. Stars ste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and has a high melting point. Thorium is an electropositive actinide whose chemistry is dominated by the +4 oxidation state; it is quite reactive and can ignite in air when finely divided. All known thorium isotopes are unstable. The most stable isotope, 232Th, has a half-life of 14.05 billion years, or about the age of the universe; it decays very slowly via alpha decay, starting a decay chain named the thorium series that ends at stable 208 Pb. On Earth, thorium and uranium are the only elements with no stable or nearly-stable isotopes that still occur naturally in large quantities as primordial elements. Thorium is estimated to be over three times as abundant as uranium in the Earth's crust, and is chiefly refined from monazite sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium radioactive decay, radioactively decays, usually by emitting an alpha particle. The half-life of this decay varies between 159,200 and 4.5 billion years for different isotopes of uranium, isotopes, making them useful for dating the age of the Earth. The most common isotopes in natural uranium are uranium-238 (which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons). Uranium has the highest atomic weight of the primordial nuclide, primordially occurring elements. Its density is about 70% higher than that of lead and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few Parts-per notation#Parts-per expressions, parts per million in soil, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemical element), but different nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have similar chemical properties, they have different atomic masses and physical properties. The term isotope is derived from the Greek roots isos (wikt:ἴσος, ἴσος "equal") and topos (wikt:τόπος, τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd (doctor), Margaret Todd in a 1913 suggestion to the British chemist Frederick Soddy, who popularized the term. The number of protons within the atomic nuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Earth
Earth is the third planet from the Sun and the only astronomical object known to Planetary habitability, harbor life. This is enabled by Earth being an ocean world, the only one in the Solar System sustaining liquid surface water. Almost all of Earth's water is contained in its global ocean, covering Water distribution on Earth, 70.8% of Earth's crust. The remaining 29.2% of Earth's crust is land, most of which is located in the form of continental landmasses within Earth's land hemisphere. Most of Earth's land is at least somewhat humid and covered by vegetation, while large Ice sheet, sheets of ice at Polar regions of Earth, Earth's polar polar desert, deserts retain more water than Earth's groundwater, lakes, rivers, and Water vapor#In Earth's atmosphere, atmospheric water combined. Earth's crust consists of slowly moving tectonic plates, which interact to produce mountain ranges, volcanoes, and earthquakes. Earth's outer core, Earth has a liquid outer core that generates a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |