|
Elimination Reactions
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group, a third type of reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Ei mechanism. E2 mechanism The E2 mechanism, where E2 stands for bimolecular elimination, involves a one-step mechanism in which ''carbon-hydrogen'' and ''carbon-halogen'' bonds break to form a double bond (''C=C Pi bond''). The specifics of the reaction are as follows: * E2 is a single step elimination, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synperiplanar
In chemistry, rotamers are chemical species that differ from one another primarily due to rotations about one or more single bonds. Various arrangements of atoms in a molecule that differ by rotation about single bonds can also be referred to as conformations. Conformers/rotamers differ little in their energies, so they are almost never separable in a practical sense. Rotations about single bonds are subject to small energy barriers. When the time scale for interconversion is long enough for isolation of individual rotamers (usually arbitrarily defined as a half-life of interconversion of 1000 seconds or longer), the species are termed atropisomers (''see:'' atropisomerism). The ring-flip of substituted cyclohexanes constitutes a common form of conformers. The study of the energetics of bond rotation is referred to as conformational analysis. In some cases, conformational analysis can be used to predict and explain product selectivity, mechanisms, and rates of reactions. Conform ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionization
Ionization or ionisation is the process by which an atom or a molecule acquires a negative or positive Electric charge, charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules, electrons, positrons, protons, antiprotons, and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Uses Everyday examples of gas ionization occur within a fluorescent lamp or other electrical discharge lamps. It is also used in radiation detectors such as the Geiger- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Bromide
Potassium bromide ( K Br) is a salt, widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to 1975 in the US. Its action is due to the bromide ion ( sodium bromide is equally effective). Potassium bromide is used as a veterinary drug, in antiepileptic medication for dogs. Under standard conditions, potassium bromide is a white crystalline powder. It is freely soluble in water; it is not soluble in acetonitrile. In a dilute aqueous solution, potassium bromide tastes sweet, at higher concentrations it tastes bitter, and tastes salty when the concentration is even higher. These effects are mainly due to the properties of the potassium ion—sodium bromide tastes salty at any concentration. In high concentration, potassium bromide strongly irritates the gastric mucous membrane, causing nausea and sometimes vomiting (a typical effect of all soluble potassium salts). Chemical properties Potassium bromide, a typ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isobutene
Isobutylene (or 2-methylpropene) is a hydrocarbon with the chemical formula . It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value. Production Polymer and chemical grade isobutylene is typically obtained by dehydrating tertiary butyl alcohol (TBA) or catalysis, catalytic dehydrogenation of isobutane.. Gasoline additives methyl tert-butyl ether (MTBE) and ethyl tert-butyl ether (ETBE), respectively, are produced by reacting methanol or ethanol with isobutylene contained in butene streams from olefin steam crackers or refineries, or with isobutylene from dehydrated TBA. Isobutylene is not isolated from the olefin or refinery butene stream before the reaction, as separating the ethers from the remaining butenes is simpler. Isobutylene can also be produced in high purities by "back-cracking" MTBE or ETBE at high temperatures and then separating the isobutylene by distillation from m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Ethoxide
Potassium ethoxide, also known as potassium ethanolate, is an off-white or yellow powder with the chemical formula of C2H5KO. Potassium ethoxide contains an ethoxide ion, the conjugate base of ethanol, which makes these compounds strongly basic. It hydrolyzes to yield ethanol and potassium hydroxide. Uses Potassium ethoxide is used as a strong base, similar to sodium methoxide, sodium and potassium methoxides, and potassium tert-butoxide. Catalytic amounts of potassium ethoxide in ethanol can be used to perform transesterification Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. Strong acids catalyze the r ... reactions that yield ethyl esters. Sodium or potassium ethoxide is also a suitable base for the malonic ester synthesis where diethyl malonate is used, since any transesterification reaction does not r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isobutyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane. The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: * If it connects at one of the two terminal carbon atoms, it is normal butyl or ''n''-butyl: (preferred IUPAC name: butyl) * If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or ''sec''-butyl: (preferred IUPAC name: butan-2-yl) The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: * If it connects at one of the three terminal carbons, it is isobutyl: (preferred IUPAC name: 2-methylpropyl) * If it connects at the central carbon, it is tertiary butyl, ''tert''-butyl or ''t''-butyl: (preferred IUPAC name: ''tert''-butyl) Nomenclature According to IUPAC nomenclature, "isobutyl", "''sec''-butyl", and "''tert''- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E2 Elimination Reaction
E, or e, is the fifth letter and the second vowel letter of the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''e'' (pronounced ); plural ''es'', ''Es'', or ''E's''. It is the most commonly used letter in many languages, including Czech, Danish, Dutch, English, French, German, Hungarian, Latin, Latvian, Norwegian, Spanish, and Swedish. Name In English, the name of the letter is the "long E" sound, pronounced . In most other languages, its name matches the letter's pronunciation in open syllables. History The Latin letter 'E' differs little from its source, the Greek letter epsilon, 'Ε'. This in turn comes from the Semitic letter '' hê'', which has been suggested to have started as a praying or calling human figure (''hillul'', 'jubilation'), and was most likely based on a similar Egyptian hieroglyph that indicated a different pronunciation. In Semitic, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
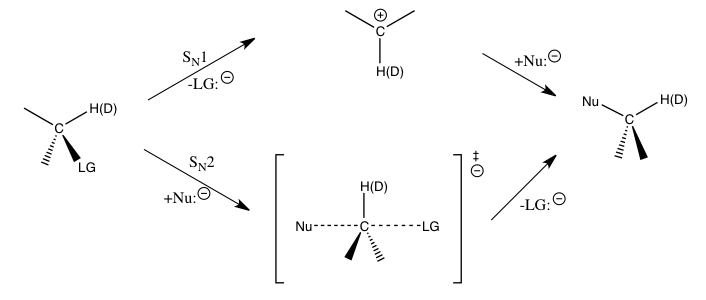
SN2 Reaction
The bimolecular nucleophilic substitution (SN2) is a type of reaction mechanism that is common in organic chemistry. In the SN2 reaction, a strong nucleophile forms a new bond to an sp3-hybridised carbon atom via a backside attack, all while the leaving group detaches from the reaction center in a concerted (i.e. simultaneous) fashion. The name SN2 refers to the Hughes-Ingold symbol of the mechanism: "SN" indicates that the reaction is a nucleophilic substitution, and "2" that it proceeds via a bimolecular mechanism, which means both the reacting species are involved in the rate-determining step. What distinguishes SN2 from the other major type of nucleophilic substitution, the SN1 reaction, is that the displacement of the leaving group, which is the rate-determining step, is separate from the nucleophilic attack in SN1. The SN2 reaction can be considered as an organic-chemistry analogue of the associative substitution from the field of inorganic chemistry. Reaction mech ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium Isotope Effect
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for the reactions involving the light (''k'') and the heavy (''k'') isotopically substituted reactants (isotopologues): KIE = ''k/k''. This change in reaction rate is a quantum effect that occurs mainly because heavier isotopologues have lower Molecular vibration, vibrational frequencies than their lighter counterparts. In most cases, this implies a greater energy input needed for heavier isotopologues to reach the transition state (or, in rare cases, Bond-dissociation energy, dissociation limit), and therefore, a slower reaction rate. The study of KIEs can help elucidate reaction mechanisms, and is occasionally exploited in drug development to improve unfavorable pharmacokinetics by protecting metabolically vulnerable C-H bonds. Background KI ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rate Determining Step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the corresponding rate equation (for comparison with the experimental rate law) is often simplified by using this approximation of the rate-determining step. In principle, the time evolution of the reactant and product concentrations can be determined from the set of simultaneous rate equations for the individual steps of the mechanism, one for each step. However, the analytical solution of these differential equations is not always easy, and in some cases numerical integration may even be required. The hypothesis of a single rate-determining step can greatly simplify the mathematics. In the simplest case the initial step is the slowest, and the overall rate is just the rate of the first step. Also, the rate equations for mechanisms with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |